LINGO-1 regulates Wnt5a signaling during neural stem and progenitor cell differentiation by modulating miR-15b-3p levels
- PMID: 34187584
- PMCID: PMC8243903
- DOI: 10.1186/s13287-021-02452-0
LINGO-1 regulates Wnt5a signaling during neural stem and progenitor cell differentiation by modulating miR-15b-3p levels
Abstract
Background: Manipulation of neural stem and progenitor cells (NSPCs) is critical for the successful treatment of spinal cord injury (SCI) by NSPC transplantation, since their differentiation into neurons and oligodendrocytes can be inhibited by factors present in inflamed myelin. In this study, we examined the effects of LINGO-1 on spinal cord-derived NSPC (sp-NSPC) differentiation, the underlying mechanisms of action, and the functional recovery of mice after transplantation of manipulated cells.
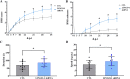
Methods: sp-NSPCs were harvested from female adult C57/BL6 mice after SCI induced with an NYU impactor. These cells were infected with lentiviral vectors containing LINGO-1 shRNA sequence or a scrambled control and transplanted into SCI mice. Tuj-1- and GFAP-positive cells were assessed by immunofluorescence staining. Wnt5a, p-JNK, JNK, and β-catenin expression was determined by Western blot and RT-qPCR. miRNAs were sequenced to detect changes in miRNA expression. Motor function was evaluated 0-35 days post-surgery by means of the Basso Mouse Scale (BMS) and by the rotarod performance test.
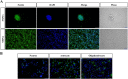
Results: We discovered that LINGO-1 shRNA increased neuronal differentiation of sp-NSPCs while decreasing astrocyte differentiation. These effects were accompanied by elevated Wnt5a protein expression, but unexpectedly, no changes in Wnt5a mRNA levels. miRNA-sequence analysis demonstrated that miR-15b-3p was a downstream mediator of LINGO-1 which suppressed Wnt5a expression. Transplantation of LINGO-1 shRNA-treated sp-NSPCs into SCI mice promoted neural differentiation, wound compaction, and motor function recovery.
Conclusions: LINGO-1 shRNA promotes neural differentiation of sp-NSPCs and Wnt5a expression, probably by downregulating miR-15b-3p. Transplantation of LINGO-1 shRNA-treated NSPCs promotes recovery of motor function after SCI, highlighting its potential as a target for SCI treatment.
Keywords: Differentiation; LINGO-1; Neural stem and progenitor cells; Spinal cord injury; Wnt5a; miR-15b-3p.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






Similar articles
-
Targeted Inhibition of Leucine-Rich Repeat and Immunoglobulin Domain-Containing Protein 1 in Transplanted Neural Stem Cells Promotes Neuronal Differentiation and Functional Recovery in Rats Subjected to Spinal Cord Injury.Crit Care Med. 2016 Mar;44(3):e146-57. doi: 10.1097/CCM.0000000000001351. Crit Care Med. 2016. PMID: 26491860
-
Lingo-1 shRNA and Notch signaling inhibitor DAPT promote differentiation of neural stem/progenitor cells into neurons.Brain Res. 2016 Mar 1;1634:34-44. doi: 10.1016/j.brainres.2015.11.029. Epub 2015 Nov 24. Brain Res. 2016. PMID: 26607252
-
Transplantation of Wnt5a-modified NSCs promotes tissue repair and locomotor functional recovery after spinal cord injury.Exp Mol Med. 2020 Dec;52(12):2020-2033. doi: 10.1038/s12276-020-00536-0. Epub 2020 Dec 14. Exp Mol Med. 2020. PMID: 33311637 Free PMC article.
-
hiPSC-Neural Stem/Progenitor Cell Transplantation Therapy for Spinal Cord Injury.Curr Stem Cell Res Ther. 2023;18(4):487-498. doi: 10.2174/1574888X17666220509222520. Curr Stem Cell Res Ther. 2023. PMID: 35538805 Review.
-
Review of transplantation of neural stem/progenitor cells for spinal cord injury.Int J Dev Neurosci. 2013 Nov;31(7):701-13. doi: 10.1016/j.ijdevneu.2013.07.004. Epub 2013 Aug 6. Int J Dev Neurosci. 2013. PMID: 23928260 Review.
Cited by
-
Transplantation of Wnt5a-modified Bone Marrow Mesenchymal Stem Cells Promotes Recovery After Spinal Cord Injury via the PI3K/AKT Pathway.Mol Neurobiol. 2024 Dec;61(12):10830-10844. doi: 10.1007/s12035-024-04248-8. Epub 2024 May 25. Mol Neurobiol. 2024. PMID: 38795301 Free PMC article.
-
The Role of the Wnt/β-Catenin Signaling Pathway in the Pathogenesis and Treatment of Spinal Cord Injury: A Review of the Latest Experimental Data.Cureus. 2025 Jul 13;17(7):e87836. doi: 10.7759/cureus.87836. eCollection 2025 Jul. Cureus. 2025. PMID: 40799869 Free PMC article. Review.
-
Wnt signaling pathway in spinal cord injury: from mechanisms to potential applications.Front Mol Neurosci. 2024 Jul 24;17:1427054. doi: 10.3389/fnmol.2024.1427054. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39114641 Free PMC article. Review.
-
Recent progress and challenges in the treatment of spinal cord injury.Protein Cell. 2023 Sep 14;14(9):635-652. doi: 10.1093/procel/pwad003. Protein Cell. 2023. PMID: 36856750 Free PMC article. Review.
-
Non-Coding RNAs in the Regulation of Hippocampal Neurogenesis and Potential Treatment Targets for Related Disorders.Biomolecules. 2022 Dec 22;13(1):18. doi: 10.3390/biom13010018. Biomolecules. 2022. PMID: 36671403 Free PMC article. Review.
References
-
- Chen Y, Cao B, Yang J, Wei QQ, Ou RW, Zhao B, Song W, Guo XY, Shang HF. Analysis and meta-analysis of five polymorphisms of the LINGO1 and LINGO2 genes in Parkinson’s disease and multiple system atrophy in a Chinese population. J Neurol. 2015;262(11):2478–2483. doi: 10.1007/s00415-015-7870-9. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

