Evaluation of the IgG antibody response to SARS CoV-2 infection and performance of a lateral flow immunoassay: cross-sectional and longitudinal analysis over 11 months
- PMID: 34187827
- PMCID: PMC8245291
- DOI: 10.1136/bmjopen-2020-048142
Evaluation of the IgG antibody response to SARS CoV-2 infection and performance of a lateral flow immunoassay: cross-sectional and longitudinal analysis over 11 months
Abstract
Objective: To evaluate the dynamics and longevity of the humoral immune response to SARS-CoV-2 infection and assess the performance of professional use of the UK-RTC AbC-19 Rapid Test lateral flow immunoassay (LFIA) for the target condition of SARS-CoV-2 spike protein IgG antibodies.
Design: Nationwide serological study.
Setting: Northern Ireland, UK, May 2020-February 2021.
Participants: Plasma samples were collected from a diverse cohort of individuals from the general public (n=279), Northern Ireland healthcare workers (n=195), pre-pandemic blood donations and research studies (n=223) and through a convalescent plasma programme (n=183). Plasma donors (n=101) were followed with sequential samples over 11 months post-symptom onset.
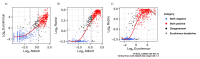
Main outcome measures: SARS-CoV-2 antibody levels in plasma samples using Roche Elecsys Anti-SARS-CoV-2 IgG/IgA/IgM, Abbott SARS-CoV-2 IgG and EuroImmun IgG SARS-CoV-2 ELISA immunoassays over time. UK-RTC AbC-19 LFIA sensitivity and specificity, estimated using a three-reference standard system to establish a characterised panel of 330 positive and 488 negative SARS-CoV-2 IgG samples.
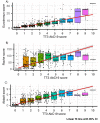
Results: We detected persistence of SARS-CoV-2 IgG antibodies for up to 10 months post-infection, across a minimum of two laboratory immunoassays. On the known positive cohort, the UK-RTC AbC-19 LFIA showed a sensitivity of 97.58% (95.28% to 98.95%) and on known negatives, showed specificity of 99.59% (98.53 % to 99.95%).
Conclusions: Through comprehensive analysis of a cohort of pre-pandemic and pandemic individuals, we show detectable levels of IgG antibodies, lasting over 46 weeks when assessed by EuroImmun ELISA, providing insight to antibody levels at later time points post-infection. We show good laboratory validation performance metrics for the AbC-19 rapid test for SARS-CoV-2 spike protein IgG antibody detection in a laboratory-based setting.
Keywords: COVID-19; diagnostic microbiology; molecular diagnostics.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- World Health Organisation . Rolling updates on coronavirus disease (COVID-19), 2020. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-a...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
