Sleep Apnea and Fetal Growth Restriction (SAFER) study: protocol for a pragmatic randomised clinical trial of positive airway pressure as an antenatal therapy for fetal growth restriction in maternal obstructive sleep apnoea
- PMID: 34187829
- PMCID: PMC8245445
- DOI: 10.1136/bmjopen-2021-049120
Sleep Apnea and Fetal Growth Restriction (SAFER) study: protocol for a pragmatic randomised clinical trial of positive airway pressure as an antenatal therapy for fetal growth restriction in maternal obstructive sleep apnoea
Abstract
Introduction: Fetal growth restriction (FGR) is a major contributor to fetal and neonatal morbidity and mortality with intrauterine, neonatal and lifelong complications. This study explores maternal obstructive sleep apnoea (OSA) as a potentially modifiable risk factor for FGR. We hypothesise that, in pregnancies complicated by FGR, treating mothers who have OSA using positive airway pressure (PAP) will improve birth weight and neonatal outcomes.
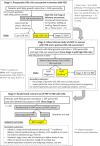
Methods and analysis: The Sleep Apnea and Fetal Growth Restriction study is a prospective, block-randomised, single-blinded, multicentre, pragmatic controlled trial. We enrol pregnant women aged 18-50, between 22 and 31 weeks of gestation, with established FGR based on second trimester ultrasound, who do not have other prespecified known causes of FGR (such as congenital anomalies or intrauterine infection). In stage 1, participants are screened by questionnaire for OSA risk. If OSA risk is identified, participants proceed to stage 2, where they undergo home sleep apnoea testing. Participants are determined to have OSA if they have an apnoea-hypopnoea index (AHI) ≥5 (if the oxygen desaturation index (ODI) is also ≥5) or if they have an AHI ≥10 (even if the ODI is <5). These participants proceed to stage 3, where they are randomised to nightly treatment with PAP or no PAP (standard care control), which is maintained until delivery. The primary outcome is unadjusted birth weight; secondary outcomes include fetal growth velocity on ultrasound, enrolment-to-delivery interval, gestational age at delivery, birth weight corrected for gestational age, stillbirth, Apgar score, rate of admission to higher levels of care (neonatal intensive care unit or special care nursery) and length of neonatal stay. These outcomes are compared between PAP and control using intention-to-treat analysis.
Ethics and dissemination: This study has been approved by the Institutional Review Boards at Washington University in St Louis, Missouri; Hadassah Hebrew University Medical Center, Jerusalem; and the University of Rochester, New York. Recruitment began in Washington University in November 2019 but stopped from March to November 2020 due to COVID-19. Recruitment began in Hadassah Hebrew University in March 2021, and in the University of Rochester in May 2021. Dissemination plans include presentations at scientific conferences and scientific publications.
Trial registration number: NCT04084990.
Keywords: maternal medicine; perinatology; sleep medicine.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
