Alternative splicing of GluN1 gates glycine site-dependent nonionotropic signaling by NMDAR receptors
- PMID: 34187890
- PMCID: PMC8271567
- DOI: 10.1073/pnas.2026411118
Alternative splicing of GluN1 gates glycine site-dependent nonionotropic signaling by NMDAR receptors
Abstract
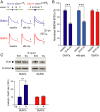
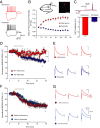
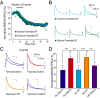
N-methyl-D-aspartate (NMDA) receptors (NMDARs), a principal subtype of excitatory neurotransmitter receptor, are composed as tetrameric assemblies of two glycine-binding GluN1 subunits and two glutamate-binding GluN2 subunits. NMDARs can signal nonionotropically through binding of glycine alone to its cognate site on GluN1. A consequence of this signaling by glycine is that NMDARs are primed such that subsequent gating, produced by glycine and glutamate, drives receptor internalization. The GluN1 subunit contains eight alternatively spliced isoforms produced by including or excluding the N1 and the C1, C2, or C2' polypeptide cassettes. Whether GluN1 alternative splicing affects nonionotropic signaling by NMDARs is a major outstanding question. Here, we discovered that glycine priming of recombinant NMDARs critically depends on GluN1 isoforms lacking the N1 cassette; glycine priming is blocked in splice variants containing N1. On the other hand, the C-terminal cassettes-C1, C2, or C2'-each permit glycine signaling. In wild-type mice, we found glycine-induced nonionotropic signaling at synaptic NMDARs in CA1 hippocampal pyramidal neurons. This nonionotropic signaling by glycine to synaptic NMDARs was prevented in mice we engineered, such that GluN1 obligatorily contained N1. We discovered in wild-type mice that, in contrast to pyramidal neurons, synaptic NMDARs in CA1 inhibitory interneurons were resistant to glycine priming. But we recapitulated glycine priming in inhibitory interneurons in mice engineered such that GluN1 obligatorily lacked the N1 cassette. Our findings reveal a previously unsuspected molecular function for alternative splicing of GluN1 in controlling nonionotropic signaling of NMDARs by activating the glycine site.
Keywords: GluN1; endocytosis; interneuron; nonionotropic; splicing.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
GluN2D-Containing N-methyl-d-Aspartate Receptors Mediate Synaptic Transmission in Hippocampal Interneurons and Regulate Interneuron Activity.Mol Pharmacol. 2016 Dec;90(6):689-702. doi: 10.1124/mol.116.105130. Epub 2016 Sep 13. Mol Pharmacol. 2016. PMID: 27625038 Free PMC article.
-
Glycine binding primes NMDA receptor internalization.Nature. 2003 Mar 20;422(6929):302-7. doi: 10.1038/nature01497. Nature. 2003. PMID: 12646920
-
Identification of a single amino acid in GluN1 that is critical for glycine-primed internalization of NMDA receptors.Mol Brain. 2013 Aug 13;6:36. doi: 10.1186/1756-6606-6-36. Mol Brain. 2013. PMID: 23941530 Free PMC article.
-
Glycine agonism in ionotropic glutamate receptors.Neuropharmacology. 2021 Aug 1;193:108631. doi: 10.1016/j.neuropharm.2021.108631. Epub 2021 May 28. Neuropharmacology. 2021. PMID: 34058193 Review.
-
Molecular basis of NMDA receptor functional diversity.Eur J Neurosci. 2011 Apr;33(8):1351-65. doi: 10.1111/j.1460-9568.2011.07628.x. Epub 2011 Mar 14. Eur J Neurosci. 2011. PMID: 21395862 Review.
Cited by
-
GluN2A-Selective NMDA Receptor Antagonists: Mimicking the U-Shaped Bioactive Conformation of TCN-201 by a [2.2]Paracyclophane System.ChemMedChem. 2022 Nov 4;17(21):e202200484. doi: 10.1002/cmdc.202200484. Epub 2022 Oct 13. ChemMedChem. 2022. PMID: 36169098 Free PMC article.
-
Two Signaling Modes Are Better than One: Flux-Independent Signaling by Ionotropic Glutamate Receptors Is Coming of Age.Biomedicines. 2024 Apr 16;12(4):880. doi: 10.3390/biomedicines12040880. Biomedicines. 2024. PMID: 38672234 Free PMC article. Review.
-
NMDA Receptor Glycine Binding Site Modulators for Prevention and Treatment of Ketamine Use Disorder.Pharmaceuticals (Basel). 2023 May 30;16(6):812. doi: 10.3390/ph16060812. Pharmaceuticals (Basel). 2023. PMID: 37375760 Free PMC article.
-
Glutamate acts on acid-sensing ion channels to worsen ischaemic brain injury.Nature. 2024 Jul;631(8022):826-834. doi: 10.1038/s41586-024-07684-7. Epub 2024 Jul 10. Nature. 2024. PMID: 38987597 Free PMC article.
-
Calcium- and calmodulin-dependent inhibition of NMDA receptor currents.Biophys J. 2024 Feb 6;123(3):277-293. doi: 10.1016/j.bpj.2023.12.018. Epub 2023 Dec 22. Biophys J. 2024. PMID: 38140727 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

