Concept for using magnetic particle imaging for intraoperative margin analysis in breast-conserving surgery
- PMID: 34188077
- PMCID: PMC8242088
- DOI: 10.1038/s41598-021-92644-8
Concept for using magnetic particle imaging for intraoperative margin analysis in breast-conserving surgery
Abstract
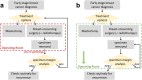
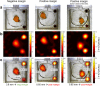
Breast-conserving surgery (BCS) is a commonly utilized treatment for early stage breast cancers but has relatively high reexcision rates due to post-surgical identification of positive margins. A fast, specific, sensitive, easy-to-use tool for assessing margins intraoperatively could reduce the need for additional surgeries, and while many techniques have been explored, the clinical need is still unmet. We assess the potential of Magnetic Particle Imaging (MPI) for intraoperative margin assessment in BCS, using a passively or actively tumor-targeted iron oxide agent and two hardware devices: a hand-held Magnetic Particle detector for identifying residual tumor in the breast, and a small-bore MPI scanner for quickly imaging the tumor distribution in the excised specimen. Here, we present both hardware systems and demonstrate proof-of-concept detection and imaging of clinically relevant phantoms.
Conflict of interest statement
The authors declare no competing interests.
Figures









References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

