Ultrasonic microbubble VEGF gene delivery improves angiogenesis of senescent endothelial progenitor cells
- PMID: 34188086
- PMCID: PMC8242093
- DOI: 10.1038/s41598-021-92754-3
Ultrasonic microbubble VEGF gene delivery improves angiogenesis of senescent endothelial progenitor cells
Abstract
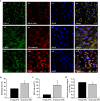
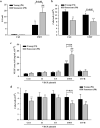
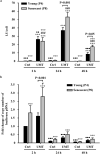
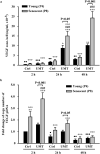
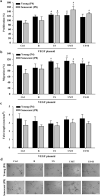
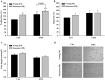
The therapeutic effects of ultrasonic microbubble transfection (UMT)-based vascular endothelial growth factor 165 (VEGF165) gene delivery on young and senescent endothelial progenitor cells (EPCs) were investigated. By UMT, plasmid DNA (pDNA) can be delivered into both young EPCs and senescent EPCs. In the UMT groups, higher pDNA-derived protein expression was found in senescent EPCs than in young EPCs. Consistent with this finding, a higher intracellular level of pDNA copy number was detected in senescent EPCs, with a peak at the 2-h time point post UMT. Ultrasonic microbubble delivery with or without VEGF improved the angiogenic properties, including the proliferation and/or migration activities, of senescent EPCs. Supernatants from young and senescent EPCs subjected to UMT-mediated VEGF transfection enhanced the proliferation and migration of human aortic endothelial cells (HAECs), and the supernatant of senescent EPCs enhanced proliferation more strongly than the supernatant from young EPCs. In the UMT groups, the stronger enhancing effect of the supernatant from senescent cells on HAEC proliferation was consistent with the higher intracellular VEGF pDNA copy number and level of protein production per cell in the supernatant from senescent cells in comparison to the supernatant from young EPCs. Given that limitations for cell therapies are the inadequate number of transplanted cells and/or insufficient cell angiogenesis, these findings provide a foundation for enhancing the therapeutic angiogenic effect of cell therapy with senescent EPCs in ischaemic cardiovascular diseases.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

