Noncytotoxic silver nanoparticles as a new antimicrobial strategy
- PMID: 34188097
- PMCID: PMC8242066
- DOI: 10.1038/s41598-021-92812-w
Noncytotoxic silver nanoparticles as a new antimicrobial strategy
Abstract
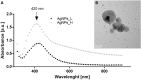
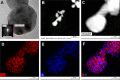
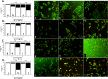
Drug-resistance of bacteria is an ongoing problem in hospital treatment. The main mechanism of bacterial virulency in human infections is based on their adhesion ability and biofilm formation. Many approaches have been invented to overcome this problem, i.e. treatment with antibacterial biomolecules, which have some limitations e.g. enzymatic degradation and short shelf stability. Silver nanoparticles (AgNPs) may be alternative to these strategies due to their unique and high antibacterial properties. Herein, we report on yeast Saccharomyces cerevisiae extracellular-based synthesis of AgNPs. Transmission electron microscopy (TEM) revealed the morphology and structure of the metallic nanoparticles, which showed a uniform distribution and good colloid stability, measured by hydrodynamic light scattering (DLS). The energy dispersive X-ray spectroscopy (EDS) of NPs confirms the presence of silver and showed that sulfur-rich compounds act as a capping agent being adsorbed on the surface of AgNPs. Antimicrobial tests showed that AgNPs inhibit the bacteria growth, while have no impact on fungi growth. Moreover, tested NPs was characterized by high inhibitory potential of bacteria biofilm formation but also eradication of established biofilms. The cytotoxic effect of the NPs on four mammalian normal and cancer cell lines was tested through the metabolic activity, cell viability and wound-healing assays. Last, but not least, ability to deep penetration of the silver colloid to the root canal was imaged by scanning electron microscopy (SEM) to show its potential as the material for root-end filling.
Conflict of interest statement
The authors declare no competing interests.
Figures



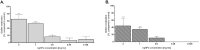



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

