Reporter cell assay for human CD33 validated by specific antibodies and human iPSC-derived microglia
- PMID: 34188106
- PMCID: PMC8242067
- DOI: 10.1038/s41598-021-92434-2
Reporter cell assay for human CD33 validated by specific antibodies and human iPSC-derived microglia
Abstract
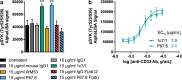
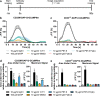
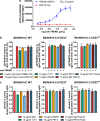
CD33/Sialic acid-binding Ig-like lectin 3 (SIGLEC3) is an innate immune receptor expressed on myeloid cells and mediates inhibitory signaling via tyrosine phosphatases. Variants of CD33 are associated with Alzheimer's disease (AD) suggesting that modulation of CD33 signaling might be beneficial in AD. Hence, there is an urgent need for reliable cellular CD33 reporter systems. Therefore, we generated a CD33 reporter cell line expressing a fusion protein consisting of the extracellular domain of either human full-length CD33 (CD33M) or the AD-protective variant CD33ΔE2 (D2-CD33/CD33m) linked to TYRO protein tyrosine kinase binding protein (TYROBP/DAP12) to investigate possible ligands and antibodies for modulation of CD33 signaling. Application of the CD33-specific antibodies P67.6 and 1c7/1 to the CD33M-DAP12 reporter cells resulted in increased phosphorylation of the kinase SYK, which is downstream of DAP12. CD33M-DAP12 but not CD33ΔE2-DAP12 expressing reporter cells showed increased intracellular calcium levels upon treatment with CD33 antibody P67.6 and partially for 1c7/1. Furthermore, stimulation of human induced pluripotent stem cell-derived microglia with the CD33 antibodies P67.6 or 1c7/1 directly counteracted the triggering receptor expressed on myeloid cells 2 (TREM2)-induced phosphorylation of SYK and decreased the phagocytic uptake of bacterial particles. Thus, the developed reporter system confirmed CD33 pathway activation by CD33 antibody clones P67.6 and 1c7/1. In addition, data showed that phosphorylation of SYK by TREM2 activation and phagocytosis of bacterial particles can be directly antagonized by CD33 signaling.
Conflict of interest statement
MM, PP, AC, LR, LP and OB are employed by a company with activities related to the topic of this manuscript. MM and OB are named inventors of a submitted patent related to upscaled production of iPSC-derived microglia and macrophages (EP20162230) that is assigned to the LIFE & BRAIN GmbH. OB and HN are named inventors on a patent related to generation of microglial precursors from pluripotent stem cells (patent family to WO2010125107A1) that is assigned to the LIFE & BRAIN GmbH and the University of Bonn. JW and OM declare no potential conflict of interest.
Figures


References
-
- Siddiqui SS, Springer SA, Verhagen A, Sundaramurthy V, Alisson-Silva F, Jiang W, Ghosh P, Varki A. The Alzheimer’s disease-protective CD33 splice variant mediates adaptive loss of function via diversion to an intracellular pool. J. Biol. Chem. 2017;292(37):15312–15320. doi: 10.1074/jbc.M117.799346. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

