Development of a bioavailable Hg(II) sensing system based on MerR-regulated visual pigment biosynthesis
- PMID: 34188121
- PMCID: PMC8242042
- DOI: 10.1038/s41598-021-92878-6
Development of a bioavailable Hg(II) sensing system based on MerR-regulated visual pigment biosynthesis
Abstract
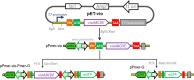
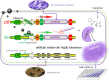
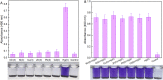
Engineered microorganisms have proven to be a highly effective and robust tool to specifically detect heavy metals in the environment. In this study, a highly specific pigment-based whole-cell biosensor has been investigated for the detection of bioavailable Hg(II) based on an artificial heavy metal resistance operon. The basic working principle of biosensors is based on the violacein biosynthesis under the control of mercury resistance (mer) promoter and mercury resistance regulator (MerR). Engineered biosensor cells have been demonstrated to selectively respond to Hg(II), and the specific response was not influenced by interfering metal ions. The response of violacein could be recognized by the naked eye, and the time required for the maximum response of violacein (5 h) was less than that of enhanced green fluorescence protein (eGFP) (8 h) in the single-signal output constructs. The response of violacein was almost unaffected by the eGFP in a double-promoter controlled dual-signals output construct. However, the response strength of eGFP was significantly decreased in this genetic construct. Exponentially growing violacein-based biosensor detected concentrations as low as 0.39 μM Hg(II) in a colorimetric method, and the linear relationship was observed in the concentration range of 0.78-12.5 μM. Non-growing biosensor cells responded to concentrations as low as 0.006 μM Hg(II) in a colorimetric method and in a Hg(II) containing plate sensitive assay, and the linear relationship was demonstrated in a very narrow concentration range. The developed biosensor was finally validated for the detection of spiked bioavailable Hg(II) in environmental water samples.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

