Bevacizumab improves tumor infiltration of mature dendritic cells and effector T-cells in triple-negative breast cancer patients
- PMID: 34188163
- PMCID: PMC8242049
- DOI: 10.1038/s41698-021-00197-w
Bevacizumab improves tumor infiltration of mature dendritic cells and effector T-cells in triple-negative breast cancer patients
Abstract
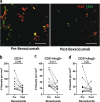
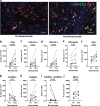
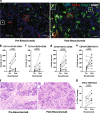
A single dose of bevacizumab reduced the density of angiopoietin-2-positive vessels while improving the infiltration of CD4+ T and CD8+ T cells, and mature dendritic cells in patients with primary triple-negative breast cancer. Our findings provide a rationale for including bevacizumab during neoadjuvant treatment to enhance the efficacy of immune checkpoint blockers in this disease.
Conflict of interest statement
R.K.J. received honorarium from Amgen; consultant fees from Elpis, Merck, Ophthotech, Pfizer, SPARC, SynDevRx, XTuit; owns equity in Enlight, Ophthotech, SynDevRx; and serves on the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, Tekla World Healthcare Fund, and received a grant from Boehringer Ingelheim. Neither any reagent nor any funding from these organizations was used in this study. DGD received consultant fees from Bayer, Simcere, Surface Oncology and BMS and research grants from Bayer, Exelixis, and BMS. SJR receives research funding from Merck, Bristol Myers Squibb, Affimed, and KITE/Gilead. SJR is a member of the scientific advisory board for Immunitas. EG is currently employed by Bristol-Myers Squibb. FSH reports grants, personal fees, and other agreements from Bristol-Myers Squibb and Novartis; personal fees from Merck, EMD Serono, Surface, Compass Therapeutics, Apricity, Aduro, Sanofi, Pionyr, 7 Hills Pharma, Verastem, Torque, Rheos, Kairos, Bicara, Psioxus Therapeutics, Eisai and Checkpoint Therapeutics; agreement with Pieris Pharmaceutical, Zumutor and Corner Therapeutics, outside the submitted work; In addition, FSH has a patent Methods for Treating MICA-Related Disorders (#20100111973) with royalties paid, a patent on Tumor antigens and uses thereof (#7250291) issued, a patent Angiopoiten-2 Biomarkers Predictive of Anti-immune checkpoint response (#20170248603) pending, a patent Compositions and Methods for Identification, Assessment, Prevention, and Treatment of Melanoma using PD-L1 Isoforms (#20160340407) pending, a patent Therapeutic peptides (#20160046716) pending, a patent Therapeutic Peptides (#20140004112) pending, a patent Therapeutic Peptides (#20170022275) pending, a patent Therapeutic Peptides (#20170008962) pending, a patent Therapeutic Peptides Patent number: 9402905 issued, a patent Methods of using Pembrolizumab and Trebananib pending, a patent Vaccine compositions and methods for restoring NKG2D pathway function against cancers Patent number: 10279021 issued, a patent antibodies that bind to MHC class I polypeptide-related sequence A Patent number: 10106611 issued, and a patent Anti-galectin Antibody Biomarkers Predictive of Anti-Immune Checkpoint and Anti-Angiogenesis Responses Publication number: 20170343552 pending. Neither any reagent nor any funding from the companies listed above was used in this study. The remaining authors declare no competing interests.
Figures
References
-
- Cortes J, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–1828. doi: 10.1016/S0140-6736(20)32531-9. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

