Attention expedites target selection by prioritizing the neural processing of distractor features
- PMID: 34188169
- PMCID: PMC8242025
- DOI: 10.1038/s42003-021-02305-9
Attention expedites target selection by prioritizing the neural processing of distractor features
Abstract
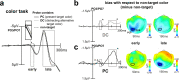
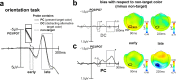
Whether doing the shopping, or driving the car - to navigate daily life, our brain has to rapidly identify relevant color signals among distracting ones. Despite a wealth of research, how color attention is dynamically adjusted is little understood. Previous studies suggest that the speed of feature attention depends on the time it takes to enhance the neural gain of cortical units tuned to the attended feature. To test this idea, we had human participants switch their attention on the fly between unpredicted target color alternatives, while recording the electromagnetic brain response to probes matching the target, a non-target, or a distracting alternative target color. Paradoxically, we observed a temporally prioritized processing of distractor colors. A larger neural modulation for the distractor followed by its stronger attenuation expedited target identification. Our results suggest that dynamic adjustments of feature attention involve the temporally prioritized processing and elimination of distracting feature representations.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

