COVID-19: biologic and immunosuppressive therapy in gastroenterology and hepatology
- PMID: 34188251
- PMCID: PMC8239481
- DOI: 10.1038/s41575-021-00480-y
COVID-19: biologic and immunosuppressive therapy in gastroenterology and hepatology
Abstract
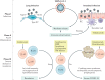
The coronavirus disease 2019 (COVID-19) pandemic is an ongoing global health crisis causing major challenges for clinical care in patients with gastrointestinal diseases. Although triggering of anti-viral immune responses is essential for clearance of infection, some patients have severe lung inflammation and multiorgan failure due to marked immune cell dysregulation and cytokine storm syndrome. Importantly, the activation of cytotoxic follicular helper T cells and a reduction of regulatory T cells have a crucial, negative prognostic role. These findings lead to the question of whether immunosuppressive and biologic therapies for gastrointestinal diseases affect the incidence or prognosis of COVID-19 and, thus, whether they should be adjusted to prevent or affect the course of the disease. In this Review, data on the use of such therapies are discussed with a primary focus on inflammatory bowel disease, autoimmune hepatitis and liver transplantation. In particular, the roles of corticosteroids, classic immunosuppressive agents (such as thiopurines and mycophenolate mofetil), small molecules (such as Janus kinase (JAK) inhibitors), and biologic agents (such as tumour necrosis factor (TNF) blockers, vedolizumab and ustekinumab) are reviewed. Finally, the use of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines for the prevention of infection in patients with gastrointestinal diseases and concomitant immunosuppressive or biologic therapy will be discussed.
© 2021. Springer Nature Limited.
Conflict of interest statement
M.F.N. has served as an advisor for Abbvie, Pentax, Giuliani, MSD, Takeda, Roche and Boehringer.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

