Targeting glioma cells by antineoplastic activity of reversine
- PMID: 34188712
- PMCID: PMC8227489
- DOI: 10.3892/ol.2021.12871
Targeting glioma cells by antineoplastic activity of reversine
Abstract
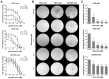
Gliomas are the most common type of primary central nervous system tumors and despite great advances in understanding the molecular basis of the disease very few new therapies have been developed. Reversine, a synthetic purine analog, is a multikinase inhibitor that targets aurora kinase A (AURKA) and aurora kinase B (AURKB). In gliomas, a high expression of AURKA or AURKB is associated with a malignant phenotype and a poor prognosis. The present study investigated reversine-related cellular and molecular antiglioma effects in HOG, T98G and U251MG cell lines. Gene and protein expression were assessed by reverse transcription-quantitative PCR and western blotting, respectively. For functional assays, human glioma cell lines (HOG, T98G and U251MG) were exposed to increasing concentrations of reversine (0.4-50 µM) and subjected to various cellular and molecular assays. Reversine reduced the viability and clonogenicity in a dose- and/or time-dependent manner in all glioma cells, with HOG (high AURKB-expression) and T98G (high AURKA-expression) cells being more sensitive compared with U251MG cells (low AURKA- and AURKB-expression). Notably, HOG cells presented higher levels of polyploidy, while T98G presented multiple mitotic spindles, which is consistent with the main regulatory functions of AURKB and AURKA, respectively. In molecular assays, reversine reduced AURKA and/or AURKB expression/activity and increased DNA damage and apoptosis markers, but autophagy-related proteins were not modulated. In conclusion, reversine potently induced mitotic catastrophe and apoptosis in glioma cells and higher basal levels of aurora kinases and genes responsive to DNA damage and may predict improved antiglioma responses to the drug. Reversine may be a potential novel drug in the antineoplastic arsenal against gliomas.
Keywords: apoptosis; aurora kinases; glioblastoma; gliomas; reversine.
Copyright: © Hirakata et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
