Exploiting the CRISPR-Cas9 gene-editing system for human cancers and immunotherapy
- PMID: 34188916
- PMCID: PMC8219901
- DOI: 10.1002/cti2.1286
Exploiting the CRISPR-Cas9 gene-editing system for human cancers and immunotherapy
Abstract
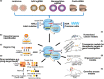
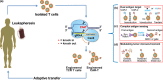
The discovery of clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9 (CRISPR-Cas9) technology has brought advances in the genetic manipulation of eukaryotic cells, which has revolutionised cancer research and treatment options. It is increasingly being used in cancer immunotherapy, including adoptive T and natural killer (NK) cell transfer, secretion of antibodies, cytokine stimulation and overcoming immune checkpoints. CRISPR-Cas9 technology is used in autologous T cells and NK cells to express various innovative antigen designs and combinations of chimeric antigen receptors (CARs) targeted at specific antigens for haematological and solid tumors. Additionally, advanced engineering in immune cells to enhance their sensing circuits with sophisticated functionality is now possible. Intensive research on the CRISPR-Cas9 system has provided scientists with the ability to overcome the hostile tumor microenvironment and generate more products for future clinical use, especially off-the-shelf, universal cellular products, bringing exciting milestones for immunotherapy. This review discussed the application and challenges of CRISPR technology in cancer research and immunotherapy, its advances and prospects for promoting new cell-based therapeutic beyond immune oncology.
Keywords: CRISPR‐Cas9; T cells; cancer; genetic manipulation; immunotherapy; natural killer cells.
© 2021 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Cooper GM. The Cell: A Molecular Approach. 2nd edition. Sunderland (MA): Sinauer Associates; 2000. The Development and Causes of Cancer. NCBI Bookshelf 2000. Available from: https://www.ncbi.nlm.nih.gov/books/NBK9963/
-
- Batool A, Malik F, Andrabi KI. Expansion of the CRISPR/Cas genome‐sculpting toolbox: innovations, applications and challenges. Mol Diagn Ther 2021; 25: 41–57. - PubMed
-
- Zhang B. CRISPR/Cas gene therapy. J Cell Physiol 2021; 236: 2459–2481. - PubMed
-
- Gruzdev A, Scott GJ, Hagler TB et al. CRISPR/Cas9‐assisted genome editing in murine embryonic stem cells. Methods Mol Biol 2019; 1960: 1–21. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
