Infant functional networks are modulated by state of consciousness and circadian rhythm
- PMID: 34189380
- PMCID: PMC8233111
- DOI: 10.1162/netn_a_00194
Infant functional networks are modulated by state of consciousness and circadian rhythm
Abstract
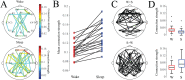
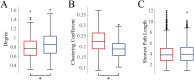
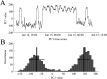
Functional connectivity networks are valuable tools for studying development, cognition, and disease in the infant brain. In adults, such networks are modulated by the state of consciousness and the circadian rhythm; however, it is unknown if infant brain networks exhibit similar variation, given the unique temporal properties of infant sleep and circadian patterning. To address this, we analyzed functional connectivity networks calculated from long-term EEG recordings (average duration 20.8 hr) from 19 healthy infants. Networks were subject specific, as intersubject correlations between weighted adjacency matrices were low. However, within individual subjects, both sleep and wake networks were stable over time, with stronger functional connectivity during sleep than wakefulness. Principal component analysis revealed the presence of two dominant networks; visual sleep scoring confirmed that these corresponded to sleep and wakefulness. Lastly, we found that network strength, degree, clustering coefficient, and path length significantly varied with time of day, when measured in either wakefulness or sleep at the group level. Together, these results suggest that modulation of healthy functional networks occurs over ∼24 hr and is robust and repeatable. Accounting for such temporal periodicities may improve the physiological interpretation and use of functional connectivity analysis to investigate brain function in health and disease.
Keywords: Cross-correlation; Electroencephalography; Functional connectivity; Graph theory; Pediatrics; Resting-state networks.
© 2021 Massachusetts Institute of Technology.
Figures




References
-
- Aeschbach, D., Matthews, J. R., Postolache, T. T., Jackson, M. A., Giesen, H. A., & Wehr, T. A. (1999). Two circadian rhythms in the human electroencephalogram during wakefulness. American Journal of Physiology, 277(6), 1771–1779. https://doi.org/10.1152/ajpregu.1999.277.6.R1771, 10600925 - DOI - PubMed
-
- Anastasiadou, M., Hadjipapas, A., Christodoulakis, M., Papathanasiou, E. S., Papacostas, S. S., & Mitsis, G. D. (2016). Epileptic seizure onset correlates with long term EEG functional brain network properties. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, 2016-Octob, 2822–2825. 10.1109/EMBC.2016.7591317 - DOI - PubMed
-
- Anastasiadou, M. N., Christodoulakis, M., Papathanasiou, E. S., Papacostas, S. S., Hadjipapas, A., & Mitsis, G. D. (2019). Graph theoretical characteristics of EEG-based functional brain networks in patients with epilepsy: The effect of reference choice and volume conduction. Frontiers in Neuroscience, 13(March), 1–18. https://doi.org/10.3389/fnins.2019.00221, 30949021 - DOI - PMC - PubMed
-
- Antoniou, I. E., & Tsompa, E. T. (2008). Statistical analysis of weighted networks. Discrete Dynamics in Nature and Society, 2008. 10.1155/2008/375452 - DOI
-
- Barkovich, A. J., Miller, S. P., Bartha, A., Newton, N., Hamrick, S. E. G., Mukherjee, P., … Vigneron, D. B. (2006). MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. American Journal of Neuroradiology, 27(3), 533–547. 16551990 - PMC - PubMed
