Effects of dietary live yeast supplementation on growth performance and biomarkers of metabolism and inflammation in heat-stressed and nutrient-restricted pigs
- PMID: 34189415
- PMCID: PMC8223600
- DOI: 10.1093/tas/txab072
Effects of dietary live yeast supplementation on growth performance and biomarkers of metabolism and inflammation in heat-stressed and nutrient-restricted pigs
Abstract
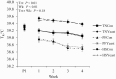
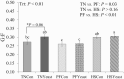
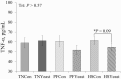
Study objectives were to determine the effects of dietary live yeast (Saccharomyces cerevisiae strain CNCM I-4407; ActisafHR+; 0.25g/kg of feed; Phileo by Lesaffre, Milwaukee, WI) on growth performance and biomarkers of metabolism and inflammation in heat-stressed and nutrient-restricted pigs. Crossbred barrows (n = 96; 79 ± 1 kg body weight [BW]) were blocked by initial BW and randomly assigned to one of six dietary-environmental treatments: 1) thermoneutral (TN) and fed ad libitum the control diet (TNCon), 2) TN and fed ad libitum a yeast containing diet (TNYeast), 3) TN and pair-fed (PF) the control diet (PFCon), 4) TN and PF the yeast containing diet (PFYeast), 5) heat stress (HS) and fed ad libitum the control diet (HSCon), or 6) HS and fed ad libitum the yeast diet (HSYeast). Following 5 d of acclimation to individual pens, pigs were enrolled in two experimental periods (P). During P1 (7 d), pigs were housed in TN conditions (20 °C) and fed their respective dietary treatments ad libitum. During P2 (28 d), HSCon and HSYeast pigs were fed ad libitum and exposed to progressive cyclical HS (28-33 °C) while TN and PF pigs remained in TN conditions and were fed ad libitum or PF to their HSCon and HSYeast counterparts. Pigs exposed to HS had an overall increase in rectal temperature, skin temperature, and respiration rate compared to TN pigs (0.3 °C, 5.5 °C, and 23 breaths per minute, respectively; P < 0.01). During P2, average daily feed intake (ADFI) decreased in HS compared to TN pigs (30%; P < 0.01). Average daily gain and final BW decreased in HS relative to TN pigs (P < 0.01); however, no differences in feed efficiency (G:F) were observed between HS and TN treatments (P > 0.16). A tendency for decreased ADFI and increased G:F was observed in TNYeast relative to TNCon pigs (P < 0.10). Circulating insulin was similar between HS and TN pigs (P > 0.42). Triiodothyronine and thyroxine levels decreased in HS compared to TN treatments (~19% and 20%, respectively; P < 0.05). Plasma tumor necrosis factor-alpha (TNF-α) did not differ across treatments (P > 0.57) but tended to decrease in HSYeast relative to HSCon pigs (P = 0.09). In summary, dietary live yeast did not affect body temperature indices or growth performance and had minimal effects on biomarkers of metabolism; however, it tended to improve G:F under TN conditions and tended to reduce the proinflammatory mediator TNF-α during HS. Further research on the potential role of dietary live yeast in pigs during HS or nutrient restriction scenarios is warranted.
Keywords: growth performance; inflammation; live yeast; swine.
© The Author(s) 2021. Published by Oxford University Press on behalf of the American Society of Animal Science.
Figures

References
-
- Abuajamieh, M., Kvidera S. K., Mayorga E. J., Kaiser A., Lei S., Seibert J. T., Horst E. A., Sanz Fernandez M. V., Ross J. W., Selsby J. T., . et al. 2018. The effect of recovery from heat stress on circulating bioenergetics and inflammatory biomarkers. J. Anim. Sci. 96:4599–4610. doi:10.1089/jas/sky345 - DOI - PMC - PubMed
-
- Beede, D. K. and Collier R. J.. . 1986. Potential nutritional strategies for intensively managed cattle during thermal stress. J. Anim. Sci. 62:543–554.
LinkOut - more resources
Full Text Sources
