Antiviral effect of high-dose ivermectin in adults with COVID-19: A proof-of-concept randomized trial
- PMID: 34189446
- PMCID: PMC8225706
- DOI: 10.1016/j.eclinm.2021.100959
Antiviral effect of high-dose ivermectin in adults with COVID-19: A proof-of-concept randomized trial
Erratum in
-
Corrigendum to Antiviral effect of high-dose ivermectin in adults with COVID-19: A proof-of-concept randomized trial [EClinicalMedicine 37 (2021) 100,959]".EClinicalMedicine. 2021 Sep;39:101119. doi: 10.1016/j.eclinm.2021.101119. Epub 2021 Aug 26. EClinicalMedicine. 2021. PMID: 34462733 Free PMC article.
Abstract
Background: There are limited antiviral options for the treatment of patients with COVID-19. Ivermectin (IVM), a macrocyclic lactone with a wide anti-parasitary spectrum, has shown potent activity against SARS-CoV-2 in vitro. This study aimed at assessing the antiviral effect of IVM on viral load of respiratory secretions and its relationship with drug concentrations in plasma.
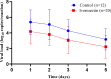
Methods: Proof-of-concept, pilot, randomized, controlled, outcome-assessor blinded trial to evaluate antiviral activity of high-dose IVM in 45 COVID-19 hospitalized patients randomized in a 2:1 ratio to standard of care plus oral IVM at 0·6 mg/kg/day for 5 days versus standard of care in 4 hospitals in Argentina. Eligible patients were adults with RT-PCR confirmed SARS-CoV-2 infection within 5 days of symptoms onset. The primary endpoint was the difference in viral load in respiratory secretions between baseline and day-5, by quantitative RT-PCR. Concentrations of IVM in plasma were measured. Study registered at ClinicalTrials.gov: NCT04381884.
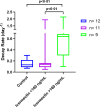
Findings: 45 participants were recruited (30 to IVM and 15 controls) between May 18 and September 9, 2020. There was no difference in viral load reduction between groups but a significant difference was found in patients with higher median plasma IVM levels (72% IQR 59-77) versus untreated controls (42% IQR 31-73) (p = 0·004). Mean ivermectin plasma concentration levels correlated with viral decay rate (r: 0·47, p = 0·02). Adverse events were similar between groups. No differences in clinical evolution at day-7 and day-30 between groups were observed.
Interpretation: A concentration dependent antiviral activity of oral high-dose IVM was identified at a dosing regimen that was well tolerated. Large trials with clinical endpoints are necessary to determine the clinical utility of IVM in COVID-19.
Funding: This work was supported by grant IP-COVID-19-625, Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación, Argentina and Laboratorio ELEA/Phoenix, Argentina.
© 2021 The Author(s).
Conflict of interest statement
AK reports grants and lecture fees from Laboratorio Elea/Phoenix and a grant from Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación, Argentina. DFA reports personal fees from Laboratorio ELEA-Phoenix outside the submitted work; RV, RS and JF report personal fees from Elea Phoenix Laboratory during the conduct of the study. MT report personal fees from Elea/Phoenix. MAT and ES are employees of Laboratorios Elea/Phoenix. SG is a member of the Board of Directors of Laboratorio Elea/Phoenix. All other authors declare no competing interests.
Figures

References
-
- National Institutes of Health . National Institutes of Health; 2020. COVID-19 treatment guidelines panel. Coronavirus disease 2019 (COVID-19) treatment guidelines.https://www.covid19treatmentguidelines.nih.gov/ Available at. Accessed 06 Oct 2020. - PubMed
-
- World Health Organization. Summary of global update on implementation of preventive chemotherapy against neglected tropical diseases in 2019. Wkly Epiemiol Rec. 2020;39(95):469–475.
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

