Shaping Up Mitochondria in Diabetic Nephropathy
- PMID: 34189465
- PMCID: PMC8238457
- DOI: 10.34067/kid.0002352020
Shaping Up Mitochondria in Diabetic Nephropathy
Abstract
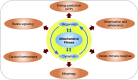
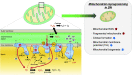
Mitochondrial medicine has experienced significant progress in recent years and is expected to grow significantly in the near future, yielding many opportunities to translate novel bench discoveries into clinical medicine. Multiple lines of evidence have linked mitochondrial dysfunction to a variety of metabolic diseases, including diabetic nephropathy (DN). Mitochondrial dysfunction presumably precedes the emergence of key histologic and biochemical features of DN, which provides the rationale to explore mitochondrial fitness as a novel therapeutic target in patients with DN. Ultimately, the success of mitochondrial medicine is dependent on a better understanding of the underlying biology of mitochondrial fitness and function. To this end, recent advances in mitochondrial biology have led to new understandings of the potential effect of mitochondrial dysfunction in a myriad of human pathologies. We have proposed that molecular mechanisms that modulate mitochondrial dynamics contribute to the alterations of mitochondrial fitness and progression of DN. In this comprehensive review, we highlight the possible effects of mitochondrial dysfunction in DN, with the hope that targeting specific mitochondrial signaling pathways may lead to the development of new drugs that mitigate DN progression. We will outline potential tools to improve mitochondrial fitness in DN as a novel therapeutic strategy. These emerging views suggest that the modulation of mitochondrial fitness could serve as a key target in ameliorating progression of kidney disease in patients with diabetes.
Conflict of interest statement
All authors have nothing to disclose.
Figures


References
-
- Skupien J, Smiles AM, Valo E, Ahluwalia TS, Gyorgy B, Sandholm N, Croall S, Lajer M, McDonnell K, Forsblom C, Harjutsalo V, Marre M, Galecki AT, Tregouet DA, Wu CY, Mychaleckyj JC, Nickerson H, Pragnell M, Rich SS, Pezzolesi MG, Hadjadj S, Rossing P, Groop PH, Krolewski AS: Variations in risk of end-stage renal disease and risk of mortality in an international study of patients with type 1 diabetes and advanced nephropathy. Diabetes Care 42: 93–101, 2019 - PMC - PubMed
-
- Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators : Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375: 323–334, 2016 - PubMed
-
- Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, Tornøe K, Zinman B, Buse JB; LEADER Steering Committee and Investigators : Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 377: 839–848, 2017 - PubMed
-
- Lane N, Martin W: The energetics of genome complexity. Nature 467: 929–934, 2010 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

