Pseudocrossidium replicatum (Taylor) R.H. Zander is a fully desiccation-tolerant moss that expresses an inducible molecular mechanism in response to severe abiotic stress
- PMID: 34189708
- PMCID: PMC8648698
- DOI: 10.1007/s11103-021-01167-3
Pseudocrossidium replicatum (Taylor) R.H. Zander is a fully desiccation-tolerant moss that expresses an inducible molecular mechanism in response to severe abiotic stress
Abstract
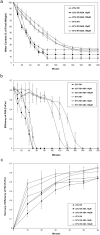
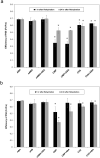
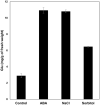
The moss Pseudocrossidium replicatum is a desiccation-tolerant species that uses an inducible system to withstand severe abiotic stress in both protonemal and gametophore tissues. Desiccation tolerance (DT) is the ability of cells to recover from an air-dried state. Here, the moss Pseudocrossidium replicatum was identified as a fully desiccation-tolerant (FDT) species. Its gametophores rapidly lost more than 90% of their water content when exposed to a low-humidity atmosphere [23% relative humidity (RH)], but abscisic acid (ABA) pretreatment diminished the final water loss after equilibrium was reached. P. replicatum gametophores maintained good maximum photosystem II (PSII) efficiency (Fv/Fm) for up to two hours during slow dehydration; however, ABA pretreatment induced a faster decrease in the Fv/Fm. ABA also induced a faster recovery of the Fv/Fm after rehydration. Protein synthesis inhibitor treatment before dehydration hampered the recovery of the Fv/Fm when the gametophores were rehydrated after desiccation, suggesting the presence of an inducible protective mechanism that is activated in response to abiotic stress. This observation was also supported by accumulation of soluble sugars in gametophores exposed to ABA or NaCl. Exogenous ABA treatment delayed the germination of P. replicatum spores and induced morphological changes in protonemal cells that resembled brachycytes. Transcriptome analyses revealed the presence of an inducible molecular mechanism in P. replicatum protonemata that was activated in response to dehydration. This study is the first RNA-Seq study of the protonemal tissues of an FDT moss. Our results suggest that P. replicatum is an FDT moss equipped with an inducible molecular response that prepares this species for severe abiotic stress and that ABA plays an important role in this response.
Keywords: ABA; Bryophyte; Dehydration; Desiccation; Gametophore; Moss; Osmotic stress; Protonema; RNA-Seq; Salt stress; Transcriptome.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Selection and Validation of Reference Genes for RT-qPCR in Protonemal Tissue of the Desiccation-Tolerant Moss Pseudocrossidium replicatum Under Multiple Abiotic Stress Conditions.Plants (Basel). 2025 Jun 7;14(12):1752. doi: 10.3390/plants14121752. Plants (Basel). 2025. PMID: 40573739 Free PMC article.
-
Desiccation tolerance in Physcomitrella patens: Rate of dehydration and the involvement of endogenous abscisic acid (ABA).Plant Cell Environ. 2018 Jan;41(1):275-284. doi: 10.1111/pce.13096. Epub 2017 Nov 29. Plant Cell Environ. 2018. PMID: 29105792
-
Acclimation and endogenous abscisic acid in the moss Physcomitrella patens during acquisition of desiccation tolerance.Physiol Plant. 2019 Nov;167(3):317-329. doi: 10.1111/ppl.12892. Epub 2019 Feb 13. Physiol Plant. 2019. PMID: 30525218
-
Novel DREB A-5 subgroup transcription factors from desert moss (Syntrichia caninervis) confers multiple abiotic stress tolerance to yeast.J Plant Physiol. 2016 May 1;194:45-53. doi: 10.1016/j.jplph.2016.02.015. Epub 2016 Mar 9. J Plant Physiol. 2016. PMID: 27016184
-
When Phased without Water: Biophysics of Cellular Desiccation, from Biomolecules to Condensates.Chem Rev. 2023 Jul 26;123(14):9010-9035. doi: 10.1021/acs.chemrev.2c00659. Epub 2023 May 3. Chem Rev. 2023. PMID: 37132487 Free PMC article. Review.
Cited by
-
Acclimation of liverwort Marchantia polymorpha to physiological drought reveals important roles of antioxidant enzymes, proline and abscisic acid in land plant adaptation to osmotic stress.PeerJ. 2021 Nov 10;9:e12419. doi: 10.7717/peerj.12419. eCollection 2021. PeerJ. 2021. PMID: 34824915 Free PMC article.
-
How the xerophytic moss Pogonatum inflexum tolerates desiccation.Plant Cell Rep. 2024 Jan 17;43(2):39. doi: 10.1007/s00299-023-03128-0. Plant Cell Rep. 2024. PMID: 38231303
-
A classification method of stress in plants using unsupervised learning algorithm and chlorophyll fluorescence technology.Front Plant Sci. 2023 Oct 23;14:1202092. doi: 10.3389/fpls.2023.1202092. eCollection 2023. Front Plant Sci. 2023. PMID: 37936937 Free PMC article.
-
Molecular biology of mosses.Plant Mol Biol. 2021 Nov;107(4-5):209-211. doi: 10.1007/s11103-021-01218-9. Epub 2021 Nov 29. Plant Mol Biol. 2021. PMID: 34843031 No abstract available.
-
Biotechnological Advances to Improve Abiotic Stress Tolerance in Crops.Int J Mol Sci. 2022 Oct 10;23(19):12053. doi: 10.3390/ijms231912053. Int J Mol Sci. 2022. PMID: 36233352 Free PMC article. Review.
References
-
- Andrews S (2010) FastQC: a quality control tool for high throughput sequence data. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
-
- Arenas-Huertero F, Arroyo A, Zhou L, Sheen J, León P. Analysis of Arabidopsis glucose insensitive mutants gin5 and gin6 reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev. 2000;14:2085–9096. doi: 10.1101/gad.14.16.2085. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

