Romosozumab improves lumbar spine bone mass and bone strength parameters relative to alendronate in postmenopausal women: results from the Active-Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk (ARCH) trial
- PMID: 34190361
- PMCID: PMC9292813
- DOI: 10.1002/jbmr.4409
Romosozumab improves lumbar spine bone mass and bone strength parameters relative to alendronate in postmenopausal women: results from the Active-Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk (ARCH) trial
Abstract
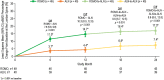
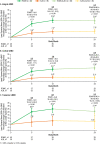
The Active-Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk (ARCH) trial (NCT01631214; https://clinicaltrials.gov/ct2/show/NCT01631214) showed that romosozumab for 1 year followed by alendronate led to larger areal bone mineral density (aBMD) gains and superior fracture risk reduction versus alendronate alone. aBMD correlates with bone strength but does not capture all determinants of bone strength that might be differentially affected by various osteoporosis therapeutic agents. We therefore used quantitative computed tomography (QCT) and finite element analysis (FEA) to assess changes in lumbar spine volumetric bone mineral density (vBMD), bone volume, bone mineral content (BMC), and bone strength with romosozumab versus alendronate in a subset of ARCH patients. In ARCH, 4093 postmenopausal women with severe osteoporosis received monthly romosozumab 210 mg sc or weekly oral alendronate 70 mg for 12 months, followed by open-label weekly oral alendronate 70 mg for ≥12 months. Of these, 90 (49 romosozumab, 41 alendronate) enrolled in the QCT/FEA imaging substudy. QCT scans at baseline and at months 6, 12, and 24 were assessed to determine changes in integral (total), cortical, and trabecular lumbar spine vBMD and corresponding bone strength by FEA. Additional outcomes assessed include changes in aBMD, bone volume, and BMC. Romosozumab caused greater gains in lumbar spine integral, cortical, and trabecular vBMD and BMC than alendronate at months 6 and 12, with the greater gains maintained upon transition to alendronate through month 24. These improvements were accompanied by significantly greater increases in FEA bone strength (p < 0.001 at all time points). Most newly formed bone was accrued in the cortical compartment, with romosozumab showing larger absolute BMC gains than alendronate (p < 0.001 at all time points). In conclusion, romosozumab significantly improved bone mass and bone strength parameters at the lumbar spine compared with alendronate. These results are consistent with greater vertebral fracture risk reduction observed with romosozumab versus alendronate in ARCH and provide insights into structural determinants of this differential treatment effect. © 2021 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: BONE MINERAL CONTENT; BONE STRENGTH; FINITE ELEMENT ANALYSIS; POSTMENOPAUSAL OSTEOPOROSIS; QUANTITATIVE COMPUTED TOMOGRAPHY.
© 2021 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Figures