Microsporidiosis in Humans
- PMID: 34190570
- PMCID: PMC8404701
- DOI: 10.1128/CMR.00010-20
Microsporidiosis in Humans
Abstract
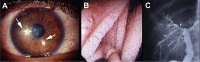
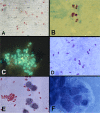
Microsporidia are obligate intracellular pathogens identified ∼150 years ago as the cause of pébrine, an economically important infection in silkworms. There are about 220 genera and 1,700 species of microsporidia, which are classified based on their ultrastructural features, developmental cycle, host-parasite relationship, and molecular analysis. Phylogenetic analysis suggests that microsporidia are related to the fungi, being grouped with the Cryptomycota as a basal branch or sister group to the fungi. Microsporidia can be transmitted by food and water and are likely zoonotic, as they parasitize a wide range of invertebrate and vertebrate hosts. Infection in humans occurs in both immunocompetent and immunodeficient hosts, e.g., in patients with organ transplantation, patients with advanced human immunodeficiency virus (HIV) infection, and patients receiving immune modulatory therapy such as anti-tumor necrosis factor alpha antibody. Clusters of infections due to latent infection in transplanted organs have also been demonstrated. Gastrointestinal infection is the most common manifestation; however, microsporidia can infect virtually any organ system, and infection has resulted in keratitis, myositis, cholecystitis, sinusitis, and encephalitis. Both albendazole and fumagillin have efficacy for the treatment of various species of microsporidia; however, albendazole has limited efficacy for the treatment of Enterocytozoon bieneusi. In addition, immune restoration can lead to resolution of infection. While the prevalence rate of microsporidiosis in patients with AIDS has fallen in the United States, due to the widespread use of combination antiretroviral therapy (cART), infection continues to occur throughout the world and is still seen in the United States in the setting of cART if a low CD4 count persists.
Keywords: AIDS; Anncaliia; Encephalitozoon; Enterocytozoon; Vittaforma; albendazole; diagnostics; fumagillin; microsporidia; prevention; therapy.
Figures




References
-
- Weiss LM. 2020. Microsporidiosis, p 825–831. In Ryan ET, Hill DR, Solomon T, Aronson N, Endy TP (ed), Hunter's tropical medicine and emerging infectious diseases. Elsevier, Amsterdam, Netherlands.
-
- Sprague V. 1977. Systematics of the microsporidia, p 1–510. In Bulla LA, Cheng TC (ed), Comparative pathobiology, vol 2. Plenum Press, New York, NY.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

