Evolutionary Pathways and Trajectories in Antibiotic Resistance
- PMID: 34190572
- PMCID: PMC8404696
- DOI: 10.1128/CMR.00050-19
Evolutionary Pathways and Trajectories in Antibiotic Resistance
Abstract
Evolution is the hallmark of life. Descriptions of the evolution of microorganisms have provided a wealth of information, but knowledge regarding "what happened" has precluded a deeper understanding of "how" evolution has proceeded, as in the case of antimicrobial resistance. The difficulty in answering the "how" question lies in the multihierarchical dimensions of evolutionary processes, nested in complex networks, encompassing all units of selection, from genes to communities and ecosystems. At the simplest ontological level (as resistance genes), evolution proceeds by random (mutation and drift) and directional (natural selection) processes; however, sequential pathways of adaptive variation can occasionally be observed, and under fixed circumstances (particular fitness landscapes), evolution is predictable. At the highest level (such as that of plasmids, clones, species, microbiotas), the systems' degrees of freedom increase dramatically, related to the variable dispersal, fragmentation, relatedness, or coalescence of bacterial populations, depending on heterogeneous and changing niches and selective gradients in complex environments. Evolutionary trajectories of antibiotic resistance find their way in these changing landscapes subjected to random variations, becoming highly entropic and therefore unpredictable. However, experimental, phylogenetic, and ecogenetic analyses reveal preferential frequented paths (highways) where antibiotic resistance flows and propagates, allowing some understanding of evolutionary dynamics, modeling and designing interventions. Studies on antibiotic resistance have an applied aspect in improving individual health, One Health, and Global Health, as well as an academic value for understanding evolution. Most importantly, they have a heuristic significance as a model to reduce the negative influence of anthropogenic effects on the environment.
Keywords: antibiotic resistance; evolutionary biology; evolutionary pathways; evolutionary trajectories; pathways; trajectories.
Figures
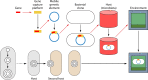
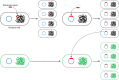
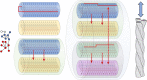

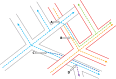


References
-
- Baquero F, Canton R. 2017. Evolutionary biology of drug resistance, p 9–32. In Mayers DL, Sobel JD, Ouellette M, Kaye KS, Marchaim D (ed), Antimicrobial drug resistance, vol 1. Mechanisms of drug resistance. Springer International Publishing, New York, NY.
-
- Ariew A. 2003. Ernst Mayr’s “ultimate/proximate” distinction reconsidered and reconstructed. Biol Philos 18:553–565. doi: 10.1023/A:1025565119032. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- CB06/02/0053/MEC | Instituto de Salud Carlos III (ISCIII)
- PI18-01942/MEC | Instituto de Salud Carlos III (ISCIII)
- PI15-00466/MEC | Instituto de Salud Carlos III (ISCIII)
- InGeMICS- B2017/BMD-3691/Consejería de Sanidad, Comunidad de Madrid
- JPIAMR2016-AC16/00043/Joint Programming Initiative on Antimicrobial Resistance (JPIAMR)
LinkOut - more resources
Full Text Sources

