Anti-Spike Protein Assays to Determine SARS-CoV-2 Antibody Levels: a Head-to-Head Comparison of Five Quantitative Assays
- PMID: 34190591
- PMCID: PMC8552734
- DOI: 10.1128/Spectrum.00247-21
Anti-Spike Protein Assays to Determine SARS-CoV-2 Antibody Levels: a Head-to-Head Comparison of Five Quantitative Assays
Abstract
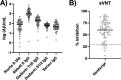
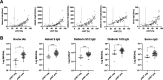
Reliable quantification of the antibody response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is highly relevant, e.g., for identifying possible vaccine failure and estimating the time of protection. Therefore, we evaluated five different anti-SARS-CoV-2 antibody assays regarding the quantification of anti-spike (S) antibodies. Sera from 69 SARS-CoV-2-naive individuals 21 ± 1 days after vaccination with a single dose of BNT162b2 (Pfizer/BioNTech) were tested using the following quantitative assays: Roche S total antibody, DiaSorin trimeric spike IgG, DiaSorin S1/S2 IgG, Abbott II IgG, and Serion/Virion IgG. Results were further compared to the percent inhibition calculated from a surrogate virus neutralization test (sVNT). Individual values were distributed over several orders of magnitude for all assays. Although the assays were in good overall agreement (ρ = 0.80 to 0.94), Passing-Bablok regression revealed systematic constant and proportional differences, which could not be eliminated by converting the results to binding antibody units (BAU) per milliliter, as suggested by the manufacturers. Seven (10%) individuals had negative sVNT results (i.e., <30% inhibition). These samples were identified by most assays and yielded significantly lower binding antibody levels. Although all assays showed good correlation, they were not interchangeable, even when converted to BAU per milliliter using the WHO international standard for SARS-CoV-2 immunoglobulin. This highlights the need for further standardization of SARS-CoV-2 serology. IMPORTANCE Reliable quantification of the antibody response to SARS-CoV-2 is highly relevant, e.g., for identifying possible vaccine failure and estimating the time of protection. We compared the performance of five CE marked tests that quantify antibodies against the viral spike protein. Our findings suggest that, although all assays showed good correlation, their results were not interchangeable, even when converted to BAU per milliliter using the WHO international standard for SARS-CoV-2 immunoglobulin. This highlights the need for further standardization of SARS-CoV-2 serology.
Keywords: SARS-CoV-2; assay standardization; comparison; immunization; quantitative antibody assays; quantitative methods; serology; vaccination.
Figures


References
-
- Abu Jabal K, Ben-Amram H, Beiruti K, Batheesh Y, Sussan C, Zarka S, Edelstein M. 2021. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill 26:2100096. doi: 10.2807/1560-7917.ES.2021.26.6.2100096. - DOI - PMC - PubMed
-
- Prendecki M, Clarke C, Brown J, Cox A, Gleeson S, Guckian M, Randell P, Pria AD, Lightstone A, Xu X-N, Barclay W, McAdoo SP, Kelleher P, Willicombe M. 2021. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet 397:1178–1181. doi: 10.1016/S0140-6736(21)00502-X. - DOI - PMC - PubMed
-
- Perkmann T, Perkmann-Nagele N, Breyer M-K, Breyer-Kohansal R, Burghuber OC, Hartl S, Aletaha D, Sieghart D, Quehenberger P, Marculescu R, Mucher P, Strassl R, Wagner OF, Binder CJ, Haslacher H. 2020. Side-by-side comparison of three fully automated SARS-CoV-2 antibody assays with a focus on specificity. Clin Chem 66:1405–1413. doi: 10.1093/clinchem/hvaa198. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

