Effect of Zuranolone vs Placebo in Postpartum Depression: A Randomized Clinical Trial
- PMID: 34190962
- PMCID: PMC8246337
- DOI: 10.1001/jamapsychiatry.2021.1559
Effect of Zuranolone vs Placebo in Postpartum Depression: A Randomized Clinical Trial
Erratum in
-
Error in Figure 3.JAMA Psychiatry. 2022 Jul 1;79(7):740. doi: 10.1001/jamapsychiatry.2022.1274. JAMA Psychiatry. 2022. PMID: 35544239 Free PMC article. No abstract available.
-
Error in Results.JAMA Psychiatry. 2023 Feb 1;80(2):191. doi: 10.1001/jamapsychiatry.2022.4181. JAMA Psychiatry. 2023. PMID: 36449311 Free PMC article. No abstract available.
Abstract
Importance: Postpartum depression (PPD) is one of the most common medical complications during and after pregnancy, negatively affecting both mother and child.
Objective: To demonstrate the efficacy and safety of zuranolone, a neuroactive steroid γ-aminobutyric acid receptor-positive allosteric modulator, in PPD.
Design, setting, and participants: This phase 3, double-blind, randomized, outpatient, placebo-controlled clinical trial was conducted between January 2017 and December 2018 in 27 enrolling US sites. Participant were women aged 18 to 45 years, 6 months or fewer post partum, with PPD (major depressive episode beginning third trimester or ≤4 weeks postdelivery), and baseline 17-item Hamilton Rating Scale for Depression (HAMD-17) score of 26 or higher. Analysis was intention to treat and began December 2018 and ended March 2019.
Interventions: Randomization 1:1 to placebo:zuranolone, 30 mg, administered orally each evening for 2 weeks.
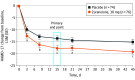
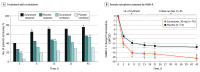
Main outcomes and measures: Primary end point was change from baseline in HAMD-17 score for zuranolone vs placebo at day 15. Secondary end points included changes from baseline in HAMD-17 total score at other time points, HAMD-17 response (≥50% score reduction) and remission (score ≤7) rates, Montgomery-Åsberg Depression Rating Scale score, and Hamilton Rating Scale for Anxiety score. Safety was assessed by adverse events and clinical assessments.
Results: Of 153 randomized patients, the efficacy set comprised 150 patients (mean [SD] age, 28.3 [5.4] years), and 148 (98.7%) completed treatment. A total of 76 patients were randomized to placebo, and 77 were randomized to zuranolone, 30 mg. Zuranolone demonstrated significant day 15 HAMD-17 score improvements from baseline vs placebo (-17.8 vs -13.6; difference, -4.2; 95% CI, -6.9 to -1.5; P = .003). Sustained differences in HAMD-17 scores favoring zuranolone were observed from day 3 (difference, -2.7; 95% CI, -5.1 to -0.3; P = .03) through day 45 (difference, -4.1; 95% CI, -6.7 to -1.4; P = .003). Sustained differences at day 15 favoring zuranolone were observed in HAMD-17 response (odds ratio, 2.63; 95% CI, 1.34-5.16; P = .005), HAMD-17 score remission (odds ratio, 2.53; 95% CI, 1.24-5.17; P = .01), change from baseline for Montgomery-Åsberg Depression Rating Scale score (difference, -4.6; 95% CI, -8.3 to -0.8; P = .02), and Hamilton Rating Scale for Anxiety score (difference, -3.9; 95% CI, -6.7 to -1.1; P = .006). One patient per group experienced a serious adverse event (confusional state in the zuranolone group and pancreatitis in the placebo group). One patient in the zuranolone group discontinued because of an adverse event vs none for placebo.
Conclusions and relevance: In this randomized clinical trial, zuranolone improved the core symptoms of depression as measured by HAMD-17 scores in women with PPD and was generally well tolerated, supporting further development of zuranolone in the treatment of PPD.
Trial registration: ClinicalTrials.gov Identifier: NCT02978326.
Conflict of interest statement
Figures
References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
-
- National Institute of Mental Health. Perinatal depression. Accessed February 8, 2021. https://www.nimh.nih.gov/health/publications/postpartum-depression-facts...
-
- Hamilton BE, Martin JA, Osterman MJK, Driscoll AK, Rossen LM. Vital Statistics Rapid Release: Births: Provisional Data for 2018: Report No. 007. National Center for Health Statistics; 2018. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

