Characteristics of Randomized Clinical Trials in Surgery From 2008 to 2020: A Systematic Review
- PMID: 34190996
- PMCID: PMC8246313
- DOI: 10.1001/jamanetworkopen.2021.14494
Characteristics of Randomized Clinical Trials in Surgery From 2008 to 2020: A Systematic Review
Abstract
Importance: Randomized clinical trials (RCTs) provide the highest level of evidence to evaluate 2 or more surgical interventions. Surgical RCTs, however, face unique challenges in design and implementation.
Objective: To evaluate the design, conduct, and reporting of contemporary surgical RCTs.
Evidence review: A literature search performed in the 2 journals with the highest impact factor in general medicine as well as 6 key surgical specialties was conducted to identify RCTs published between 2008 and 2020. All RCTs describing a surgical intervention in both experimental and control arms were included. The quality of included data was assessed by establishing an a priori protocol containing all the details to extract. Trial characteristics, fragility index, risk of bias (Cochrane Risk of Bias 2 Tool), pragmatism (Pragmatic Explanatory Continuum Indicator Summary 2 [PRECIS-2]), and reporting bias were assessed.
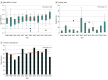
Findings: A total of 388 trials were identified. Of them, 242 (62.4%) were registered; discrepancies with the published protocol were identified in 81 (33.5%). Most trials used superiority design (329 [84.8%]), and intention-to-treat as primary analysis (221 [56.9%]) and were designed to detect a large treatment effect (50.0%; interquartile range [IQR], 24.7%-63.3%). Only 123 trials (31.7%) used major clinical events as the primary outcome. Most trials (303 [78.1%]) did not control for surgeon experience; only 17 trials (4.4%) assessed the quality of the intervention. The median sample size was 122 patients (IQR, 70-245 patients). The median follow-up was 24 months (IQR, 12.0-32.0 months). Most trials (211 [54.4%]) had some concern of bias and 91 (23.5%) had high risk of bias. The mean (SD) PRECIS-2 score was 3.52 (0.65) and increased significantly over the study period. Most trials (212 [54.6%]) reported a neutral result; reporting bias was identified in 109 of 211 (51.7%). The median fragility index was 3.0 (IQR, 1.0-6.0). Multiplicity was detected in 175 trials (45.1%), and only 35 (20.0%) adjusted for multiple comparisons.
Conclusions and relevance: In this systematic review, the size of contemporary surgical trials was small and the focus was on minor clinical events. Trial registration remained suboptimal and discrepancies with the published protocol and reporting bias were frequent. Few trials controlled for surgeon experience or assessed the quality of the intervention.
Conflict of interest statement
Figures

Comment in
-
Surgical Culture Shifts and Randomized Clinical Trials.JAMA Netw Open. 2021 Jun 1;4(6):e2115456. doi: 10.1001/jamanetworkopen.2021.15456. JAMA Netw Open. 2021. PMID: 34191001 No abstract available.
References
-
- Solomon MJ, Laxamana A, Devore L, McLeod RS. Randomized controlled trials in surgery. Surgery. 1994;115(6):707-712. - PubMed

