Longitudinal Assessment of Diagnostic Test Performance Over the Course of Acute SARS-CoV-2 Infection
- PMID: 34191025
- PMCID: PMC8448437
- DOI: 10.1093/infdis/jiab337
Longitudinal Assessment of Diagnostic Test Performance Over the Course of Acute SARS-CoV-2 Infection
Abstract
Background: Serial screening is critical for restricting spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by facilitating timely identification of infected individuals to interrupt transmission. Variation in sensitivity of different diagnostic tests at different stages of infection has not been well documented.
Methods: In a longitudinal study of 43 adults newly infected with SARS-CoV-2, all provided daily saliva and nasal swabs for quantitative reverse transcription polymerase chain reaction (RT-qPCR), Quidel SARS Sofia antigen fluorescent immunoassay (FIA), and live virus culture.
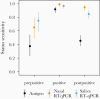
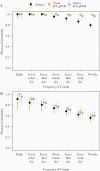
Results: Both RT-qPCR and Quidel SARS Sofia antigen FIA peaked in sensitivity during the period in which live virus was detected in nasal swabs, but sensitivity of RT-qPCR tests rose more rapidly prior to this period. We also found that serial testing multiple times per week increases the sensitivity of antigen tests.
Conclusions: RT-qPCR tests are more effective than antigen tests at identifying infected individuals prior to or early during the infectious period and thus for minimizing forward transmission (given timely results reporting). All tests showed >98% sensitivity for identifying infected individuals if used at least every 3 days. Daily screening using antigen tests can achieve approximately 90% sensitivity for identifying infected individuals while they are viral culture positive.
Keywords: RT-qPCR testing; SARS-CoV-2; antigen testing; diagnostic testing; test sensitivity.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

Update of
-
Longitudinal assessment of diagnostic test performance over the course of acute SARS-CoV-2 infection.medRxiv [Preprint]. 2021 Mar 22:2021.03.19.21253964. doi: 10.1101/2021.03.19.21253964. medRxiv. 2021. Update in: J Infect Dis. 2021 Sep 17;224(6):976-982. doi: 10.1093/infdis/jiab337. PMID: 33791719 Free PMC article. Updated. Preprint.
Comment in
-
Frequent Severe Acute Respiratory Syndrome Coronavirus 2 Antigen Testing in a Disease-Free Population.J Infect Dis. 2021 Dec 1;224(11):1986-1987. doi: 10.1093/infdis/jiab529. J Infect Dis. 2021. PMID: 34644383 No abstract available.
-
Low Sensitivity of Rapid Antigen Tests to Detect Severe Acute Respiratory Syndrome Coronavirus 2 Infections Before and on the Day of Symptom Onset in Nursing Home Staff and Residents, Germany, January-March 2021.J Infect Dis. 2021 Dec 1;224(11):1987-1989. doi: 10.1093/infdis/jiab528. J Infect Dis. 2021. PMID: 34648637 Free PMC article. No abstract available.
References
-
- Krüger LJ, Gaeddert M, Köppel L, et al. . Evaluation of the accuracy, ease of use and limit of detection of novel, rapid, antigen-detecting point-of-care diagnostics for SARS-CoV-2. medRxiv, doi: 10.1101/2020.10.01.20203836, 4. October 2020, preprint: not peer reviewed. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

