Genomic and Proteomic Study of Andreprevotia ripae Isolated from an Anthill Reveals an Extensive Repertoire of Chitinolytic Enzymes
- PMID: 34191517
- PMCID: PMC8802321
- DOI: 10.1021/acs.jproteome.1c00358
Genomic and Proteomic Study of Andreprevotia ripae Isolated from an Anthill Reveals an Extensive Repertoire of Chitinolytic Enzymes
Abstract
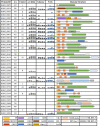
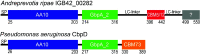
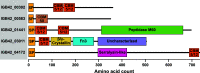
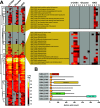
Chitin is an abundant natural polysaccharide that is hard to degrade because of its crystalline nature and because it is embedded in robust co-polymeric materials containing other polysaccharides, proteins, and minerals. Thus, it is of interest to study the enzymatic machineries of specialized microbes found in chitin-rich environments. We describe a genomic and proteomic analysis of Andreprevotia ripae, a chitinolytic Gram-negative bacterium isolated from an anthill. The genome of A. ripae encodes four secreted family GH19 chitinases of which two were detected and upregulated during growth on chitin. In addition, the genome encodes as many as 25 secreted GH18 chitinases, of which 17 were detected and 12 were upregulated during growth on chitin. Finally, the single lytic polysaccharide monooxygenase (LPMO) was strongly upregulated during growth on chitin. Whereas 66% of the 29 secreted chitinases contained two carbohydrate-binding modules (CBMs), this fraction was 93% (13 out of 14) for the upregulated chitinases, suggesting an important role for these CBMs. Next to an unprecedented multiplicity of upregulated chitinases, this study reveals several chitin-induced proteins that contain chitin-binding CBMs but lack a known catalytic function. These proteins are interesting targets for discovery of enzymes used by nature to convert chitin-rich biomass. The MS proteomic data have been deposited in the PRIDE database with accession number PXD025087.
Keywords: CBM; Chitinase; GH18; GH19; LPMO; carbohydrate-binding module; chitin; chitinolytic machineries; genome analysis; proteomics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
The Fish Pathogen Aliivibrio salmonicida LFI1238 Can Degrade and Metabolize Chitin despite Gene Disruption in the Chitinolytic Pathway.Appl Environ Microbiol. 2021 Sep 10;87(19):e0052921. doi: 10.1128/AEM.00529-21. Epub 2021 Sep 10. Appl Environ Microbiol. 2021. PMID: 34319813 Free PMC article.
-
Listeria monocytogenes has a functional chitinolytic system and an active lytic polysaccharide monooxygenase.FEBS J. 2015 Mar;282(5):921-36. doi: 10.1111/febs.13191. Epub 2015 Jan 29. FEBS J. 2015. PMID: 25565565
-
Carbohydrate-binding modules of ChiB and ChiC promote the chitinolytic system of Serratia marcescens BWL1001.Enzyme Microb Technol. 2023 Jan;162:110118. doi: 10.1016/j.enzmictec.2022.110118. Epub 2022 Aug 31. Enzyme Microb Technol. 2023. PMID: 36081184
-
Bacterial Chitinase System as a Model of Chitin Biodegradation.Adv Exp Med Biol. 2019;1142:131-151. doi: 10.1007/978-981-13-7318-3_7. Adv Exp Med Biol. 2019. PMID: 31102245 Review.
-
Chitin-Active Lytic Polysaccharide Monooxygenases.Adv Exp Med Biol. 2019;1142:115-129. doi: 10.1007/978-981-13-7318-3_6. Adv Exp Med Biol. 2019. PMID: 31102244 Review.
Cited by
-
Proteomic and Transcriptomic Analyses to Decipher the Chitinolytic Response of Jeongeupia spp.Mar Drugs. 2023 Aug 15;21(8):448. doi: 10.3390/md21080448. Mar Drugs. 2023. PMID: 37623729 Free PMC article.
-
Multi-enzyme Machinery for Chitin Degradation in the Chitinolytic Bacterium Chitiniphilus shinanonensis SAY3T.Curr Microbiol. 2023 Oct 5;80(11):360. doi: 10.1007/s00284-023-03489-5. Curr Microbiol. 2023. PMID: 37796346
-
Isolation, biochemical characterization, and genome sequencing of two high-quality genomes of a novel chitinolytic Jeongeupia species.Microbiologyopen. 2023 Aug;12(4):e1372. doi: 10.1002/mbo3.1372. Microbiologyopen. 2023. PMID: 37642486 Free PMC article.
References
-
- Mohan K.; Ganesan A. R.; Muralisankar T.; Jayakumar R.; Sathishkumar P.; Uthayakumar V.; Chandirasekar R.; Revathi N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. 10.1016/j.tifs.2020.08.016. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

