Ventral stress fibers induce plasma membrane deformation in human fibroblasts
- PMID: 34191528
- PMCID: PMC8684729
- DOI: 10.1091/mbc.E21-03-0096
Ventral stress fibers induce plasma membrane deformation in human fibroblasts
Abstract
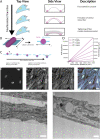
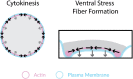
Interactions between the actin cytoskeleton and the plasma membrane are important in many eukaryotic cellular processes. During these processes, actin structures deform the cell membrane outward by applying forces parallel to the fiber's major axis (as in migration) or they deform the membrane inward by applying forces perpendicular to the fiber's major axis (as in the contractile ring during cytokinesis). Here we describe a novel actin-membrane interaction in human dermal myofibroblasts. When labeled with a cytosolic fluorophore, the myofibroblasts displayed prominent fluorescent structures on the ventral side of the cell. These structures are present in the cell membrane and colocalize with ventral actin stress fibers, suggesting that the stress fibers bend the membrane to form a "cytosolic pocket" that the fluorophores diffuse into, creating the observed structures. The existence of this pocket was confirmed by transmission electron microscopy. While dissolving the stress fibers, inhibiting fiber protein binding, or inhibiting myosin II binding of actin removed the observed pockets, modulating cellular contractility did not remove them. Taken together, our results illustrate a novel actin-membrane bending topology where the membrane is deformed outward rather than being pinched inward, resembling the topological inverse of the contractile ring found in cytokinesis.
Figures






Similar articles
-
Crosstalk between myosin II and formin functions in the regulation of force generation and actomyosin dynamics in stress fibers.Cells Dev. 2021 Dec;168:203736. doi: 10.1016/j.cdev.2021.203736. Epub 2021 Aug 26. Cells Dev. 2021. PMID: 34455135
-
Active FHOD1 promotes the formation of functional actin stress fibers.Biochem J. 2019 Oct 30;476(20):2953-2963. doi: 10.1042/BCJ20190535. Biochem J. 2019. PMID: 31657439
-
Abolishing myofibroblast arrhythmogeneicity by pharmacological ablation of α-smooth muscle actin containing stress fibers.Circ Res. 2011 Oct 28;109(10):1120-31. doi: 10.1161/CIRCRESAHA.111.244798. Epub 2011 Sep 15. Circ Res. 2011. PMID: 21921266
-
Actin stress fibre subtypes in mesenchymal-migrating cells.Open Biol. 2013 Jun 19;3(6):130001. doi: 10.1098/rsob.130001. Open Biol. 2013. PMID: 23782578 Free PMC article. Review.
-
Ras-related GTPases and the cytoskeleton.Mol Biol Cell. 1992 May;3(5):475-9. doi: 10.1091/mbc.3.5.475. Mol Biol Cell. 1992. PMID: 1611153 Free PMC article. Review.
Cited by
-
Merging of ventral fibers at adhesions drives the remodeling of cellular contractile systems in fibroblasts.Cytoskeleton (Hoboken). 2022 Sep;79(9-11):81-93. doi: 10.1002/cm.21722. Epub 2022 Sep 5. Cytoskeleton (Hoboken). 2022. PMID: 35996927 Free PMC article.
-
Interface self-referenced dynamic full-field optical coherence tomography.Biomed Opt Express. 2023 Jun 21;14(7):3491-3505. doi: 10.1364/BOE.488663. eCollection 2023 Jul 1. Biomed Opt Express. 2023. PMID: 37497503 Free PMC article.
References
-
- Abercrombie M, Heaysman JEM, Pegrum SM (1970). The locomotion of fibroblasts in culture I. Movements of the leading edge. Exp Cell Res 59, 393–398. - PubMed
-
- Abercrombie M, Heaysman JEM, Pegrum SM (1971). The locomotion of fibroblasts in culture: IV. Electron microscopy of the leading lamella. Exp Cell Res 67, 359–367. - PubMed
-
- Arpin M, Algrain M, Louvard D (1994). Membrane-actin microfilament connections: an increasing diversity of players related to band 4.1. Curr Opin Cell Biol 6, 136–141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

