Borrelia afzelii Infection in the Rodent Host Has Dramatic Effects on the Bacterial Microbiome of Ixodes ricinus Ticks
- PMID: 34191531
- PMCID: PMC8388833
- DOI: 10.1128/AEM.00641-21
Borrelia afzelii Infection in the Rodent Host Has Dramatic Effects on the Bacterial Microbiome of Ixodes ricinus Ticks
Abstract
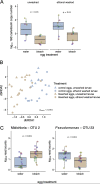
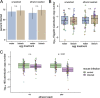
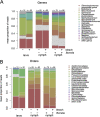
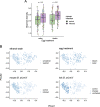
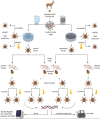
The microbiome of blood-sucking arthropods can shape their competence to acquire and maintain infections with vector-borne pathogens. We used a controlled study to investigate the interactions between Borrelia afzelii, which causes Lyme borreliosis in Europe, and the bacterial microbiome of Ixodes ricinus, its primary tick vector. We applied a surface sterilization treatment to I. ricinus eggs to produce dysbiosed tick larvae that had a low bacterial abundance and a changed bacterial microbiome compared to those of the control larvae. Dysbiosed and control larvae fed on B. afzelii-infected mice and uninfected control mice, and the engorged larvae were left to molt into nymphs. The nymphs were tested for B. afzelii infection, and their bacterial microbiome underwent 16S rRNA amplicon sequencing. Surprisingly, larval dysbiosis had no effect on the vector competence of I. ricinus for B. afzelii, as the nymphal infection prevalence and the nymphal spirochete load were the same between the dysbiosed group and the control group. The strong effect of egg surface sterilization on the tick bacterial microbiome largely disappeared once the larvae molted into nymphs. The most important determinant of the bacterial microbiome of I. ricinus nymphs was the B. afzelii infection status of the mouse on which the nymphs had fed as larvae. Nymphs that had taken their larval blood meal from an infected mouse had a less abundant but more diverse bacterial microbiome than the control nymphs. Our study demonstrates that vector-borne infections in the vertebrate host shape the microbiome of the arthropod vector. IMPORTANCE Many blood-sucking arthropods transmit pathogens that cause infectious disease. For example, Ixodes ricinus ticks transmit the bacterium Borrelia afzelii, which causes Lyme disease in humans. Ticks also have a microbiome, which can influence their ability to acquire and transmit tick-borne pathogens such as B. afzelii. We sterilized I. ricinus eggs with bleach, and the tick larvae that hatched from these eggs had a dramatically reduced and changed bacterial microbiome compared to that of control larvae. These larvae fed on B. afzelii-infected mice, and the resultant nymphs were tested for B. afzelii and for their bacterial microbiome. We found that our manipulation of the bacterial microbiome had no effect on the ability of the tick larvae to acquire and maintain populations of B. afzelii. In contrast, we found that B. afzelii infection had dramatic effects on the bacterial microbiome of I. ricinus nymphs. Our study demonstrates that infections in the vertebrate host can shape the tick microbiome.
Keywords: Borrelia afzelii; Ixodes ricinus; Lyme disease; dysbiosis; egg surface sterilization; microbiome; tick-borne disease; vector-borne disease.
Figures


References
-
- de la Fuente J, Antunes S, Bonnet S, Cabezas-Cruz A, Domingos AG, Estrada-Pena A, Johnson N, Kocan KM, Mansfield KL, Nijhof AM, Papa A, Rudenko N, Villar M, Alberdi P, Torina A, Ayllon N, Vancova M, Golovchenko M, Grubhoffer L, Caracappa S, Fooks AR, Gortazar C, Rego ROM. 2017. Tick-pathogen interactions and vector competence: identification of molecular drivers for tick-borne diseases. Front Cell Infect Microbiol 7:114. 10.3389/fcimb.2017.00114. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

