Heterogeneity within molecular subtypes of breast cancer
- PMID: 34191627
- PMCID: PMC8424677
- DOI: 10.1152/ajpcell.00109.2021
Heterogeneity within molecular subtypes of breast cancer
Abstract
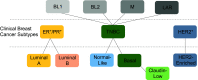
Breast cancer is the quintessential example of how molecular characterization of tumor biology guides therapeutic decisions. From the discovery of the estrogen receptor to current clinical molecular profiles to evolving single-cell analytics, the characterization and compartmentalization of breast cancer into divergent subtypes is clear. However, competing with this divergent model of breast cancer is the recognition of intratumoral heterogeneity, which acknowledges the possibility that multiple different subtypes exist within a single tumor. Intratumoral heterogeneity is driven by both intrinsic effects of the tumor cells themselves as well as extrinsic effects from the surrounding microenvironment. There is emerging evidence that these intratumoral molecular subtypes are not static; rather, plasticity between divergent subtypes is possible. Interconversion between seemingly different subtypes within a tumor drives tumor progression, metastases, and treatment resistance. Therapeutic strategies must, therefore, contend with changing phenotypes in an individual patient's tumor. Identifying targetable drivers of molecular heterogeneity may improve treatment durability and disease progression.
Keywords: cell-state heterogeneity; dynamic interconversion; hierarchical heterogeneity; intratumoral heterogeneity; plasticity.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature 406: 747–752, 2000. doi:10.1038/35021093. - DOI - PubMed
-
- Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, Quackenbush JF, Stijleman IJ, Palazzo J, Marron JS, Nobel AB, Mardis E, Nielsen TO, Ellis MJ, Perou CM, Bernard PS. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 27: 1160–1167, 2009. doi:10.1200/JCO.2008.18.1370. - DOI - PMC - PubMed
-
- Priedigkeit N, Hartmaier RJ, Chen Y, Vareslija D, Basudan A, Watters RJ, Thomas R, Leone JP, Lucas PC, Bhargava R, Hamilton RL, Chmielecki J, Puhalla SL, Davidson NE, Oesterreich S, Brufsky AM, Young L, Lee AV. Intrinsic subtype switching and acquired ERBB2/HER2 amplifications and mutations in breast cancer brain metastases. JAMA Oncol 3: 666–671, 2017. doi:10.1001/jamaoncol.2016.5630. - DOI - PMC - PubMed
-
- Garcia-Recio S, Thennavan A, East MP, Parker JS, Cejalvo JM, Garay JP, Hollern DP, He X, Mott KR, Galván P, Fan C, Selitsky SR, Coffey AR, Marron D, Brasó-Maristany F, Burgués O, Albanell J, Rojo F, Lluch A, de Dueñas EM, Rosen JM, Johnson GL, Carey LA, Prat A, Perou CM. FGFR4 regulates tumor subtype differentiation in luminal breast cancer and metastatic disease. J Clin Invest 130: 4871–4887, 2020. doi:10.1172/JCI130323. - DOI - PMC - PubMed
-
- Jordan NV, Bardia A, Wittner BS, Benes C, Ligorio M, Zheng Y, Yu M, Sundaresan TK, Licausi JA, Desai R, O'Keefe RM, Ebright RY, Boukhali M, Sil S, Onozato ML, Iafrate AJ, Kapur R, Sgroi D, Ting DT, Toner M, Ramaswamy S, Haas W, Maheswaran S, Haber DA. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature 537: 102–106, 2016. doi:10.1038/nature19328. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

