Building biorepositories in the midst of a pandemic
- PMID: 34192049
- PMCID: PMC8134891
- DOI: 10.1017/cts.2021.6
Building biorepositories in the midst of a pandemic
Abstract
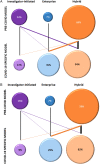
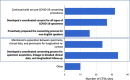
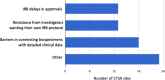
Biospecimen repositories play a vital role in enabling investigation of biologic mechanisms, identification of disease-related biomarkers, advances in diagnostic assays, recognition of microbial evolution, and characterization of new therapeutic targets for intervention. They rely on the complex integration of scientific need, regulatory oversight, quality control in collection, processing and tracking, and linkage to robust phenotype information. The COVID-19 pandemic amplified many of these considerations and illuminated new challenges, all while academic health centers were trying to adapt to unprecedented clinical demands and heightened research constraints not witnessed in over 100 years. The outbreak demanded rapid understanding of SARS-CoV-2 to develop diagnostics and therapeutics, prompting the immediate need for access to high quality, well-characterized COVID-19-associated biospecimens. We surveyed 60 Clinical and Translational Science Award (CTSA) hubs to better understand the strategies and barriers encountered in biobanking before and in response to the COVID-19 pandemic. Feedback revealed a major shift in biorepository model, specimen-acquisition and consent process from a combination of investigator-initiated and institutional protocols to an enterprise-serving strategy. CTSA hubs were well equipped to leverage established capacities and expertise to quickly respond to the scientific needs of this crisis through support of institutional approaches in biorepository management.
Keywords: Biorepository; COVID-19; CTSA; SARS-CoV-2; biobanking IRB; informed consent; regulatory; sample; specimen.
© The Association for Clinical and Translational Science 2021.
Figures
References
-
- Greenberg B, Christian J, Henry LM, et al. Biorepositories: Addendum to Registries for Evaluating Patient Outcomes: A User’s Guide, Third Edition, in Biorepositories: Addendum to Registries for Evaluating Patient Outcomes: A User’s Guide, Third Edition. Rockville, MD: 2018. - PubMed
-
- Cola P and Williams M. Biorepository considerations in a changing regulatory landscape. SRA Midwest/NE Section Meeting 2019. (https://higherlogicdownload.s3.amazonaws.com/SRAINTERNATIONAL/47435d22-1...)
-
- World Health Organization. WHO Director-General’s Opening Remarks at The Media Briefing on COVID-19-11 March 2020 [Internet] [cited Sept 28, 2020]. (https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re...)
-
- Mehrotra P, Malani P, Prashant Y. Personal Protective Equipment Shortages During COVID-19—Supply Chain–Related Causes and Mitigation Strategies. JAMA Health Forum, [Internet] 2020. (https://jamanetwork.com/channels/health-forum/fullarticle/2766118) - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
