The RADx Tech Clinical Studies Core: A Model for Academic Based Clinical Studies
- PMID: 34192287
- PMCID: PMC8112667
- DOI: 10.1109/OJEMB.2021.3070830
The RADx Tech Clinical Studies Core: A Model for Academic Based Clinical Studies
Abstract
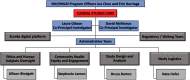
The National Institutes of Health (NIH) launched the Rapid Acceleration of Diagnostics (RADxSM) Tech initiative to support the development and commercialization of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) point-of-care test devices. The primary objective of the Clinical Studies Core (CSC) was to perform SARS-CoV-2 device studies involving diverse populations and settings. Within a few months, the infrastructure for clinical studies was developed, including a master protocol, digital study platform, data management system, single IRB, and multi-site partnerships. Data from some studies are being used to support Emergency Use Authorization of novel SARS-CoV-2 test devices. The CSC reduced the typical time and cost of developing medical devices and highlighted the impactful role of academic and NIH partnership in addressing public health needs at a rapid pace during a global pandemic. The structure, deployment, and lessons learned from this experience are widely applicable to future in vitro diagnostic device clinical studies.
Keywords: COVID-19; SARS-CoV-2; [Formula: see text] diagnostics; point-of-care testing; rapid acceleration of diagnostics.
This work is licensed under a Creative Commons Attribution 4.0 License. For more information, see https://creativecommons.org/licenses/by/4.0/.
Figures
Similar articles
-
The critical role of engineering in the rapid development of COVID-19 diagnostics: Lessons from the RADx Tech Test Verification Core.Sci Adv. 2023 Apr 7;9(14):eade4962. doi: 10.1126/sciadv.ade4962. Epub 2023 Apr 7. Sci Adv. 2023. PMID: 37027461 Free PMC article. Review.
-
The RADx Tech Deployment Core: A Model for Academic/Government Based Support of Large-Scale Manufacturing and Implementation of Medical Technologies.IEEE Open J Eng Med Biol. 2021 Apr 28;2:158-162. doi: 10.1109/OJEMB.2021.3070822. eCollection 2021 Apr 28. IEEE Open J Eng Med Biol. 2021. PMID: 34192288 Free PMC article.
-
RADx-UP Testing Core: Access to COVID-19 Diagnostics in Community-Engaged Research with Underserved Populations.J Clin Microbiol. 2023 Aug 23;61(8):e0036723. doi: 10.1128/jcm.00367-23. Epub 2023 Jul 3. J Clin Microbiol. 2023. PMID: 37395655 Free PMC article.
-
The RADxSM Tech Process: Accelerating Innovation for COVID-19 Testing.IEEE Pulse. 2021 May-Jun;12(3):21-23. doi: 10.1109/MPULS.2021.3078602. IEEE Pulse. 2021. PMID: 34156930
-
Standardizing, harmonizing, and protecting data collection to broaden the impact of COVID-19 research: the rapid acceleration of diagnostics-underserved populations (RADx-UP) initiative.J Am Med Inform Assoc. 2022 Aug 16;29(9):1480-1488. doi: 10.1093/jamia/ocac097. J Am Med Inform Assoc. 2022. PMID: 35678579 Free PMC article. Review.
Cited by
-
Challenges in Use of Practice-based Research Networks for a Medical Device Trial to Detect SARS-CoV-2.J Prim Care Community Health. 2023 Jan-Dec;14:21501319231164540. doi: 10.1177/21501319231164540. J Prim Care Community Health. 2023. PMID: 37005790 Free PMC article.
-
RADx Tech Viability and Steering Panels: A Model for MedTech Translational Grant Review.IEEE Open J Eng Med Biol. 2021;2:125-130. doi: 10.1109/ojemb.2021.3070821. Epub 2021 Apr 28. IEEE Open J Eng Med Biol. 2021. PMID: 35382011 Free PMC article.
-
Minimization of MEDA Biochip-Size in Droplet Routing.Biosensors (Basel). 2022 Apr 27;12(5):277. doi: 10.3390/bios12050277. Biosensors (Basel). 2022. PMID: 35624578 Free PMC article.
-
Design and Preliminary Findings of Adherence to the Self-Testing for Our Protection From COVID-19 (STOP COVID-19) Risk-Based Testing Protocol: Prospective Digital Study.JMIR Form Res. 2022 Jun 16;6(6):e38113. doi: 10.2196/38113. JMIR Form Res. 2022. PMID: 35649180 Free PMC article.
-
Design, creation, and use of the Test Us Bank (TUB) COVID-19 sample biorepository.BMC Infect Dis. 2024 Nov 12;24(1):1282. doi: 10.1186/s12879-024-10154-0. BMC Infect Dis. 2024. PMID: 39528968 Free PMC article.
References
-
- Walker P. G. et al., “The global impact of COVID-19 and strategies for mitigation and suppression,” Imp. Coll. COVID-19 Response Team, 2020.
-
- Hadaya J., Schumm M., and Livingston E. H., “Testing individuals for coronavirus disease 2019 (COVID-19),” JAMA, vol. 323, no. 19, p. 1981, 2020. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous