First evaluation of the safety, pharmacokinetics, and pharmacodynamics of BAY 2433334, a small molecule targeting coagulation factor XIa
- PMID: 34192419
- PMCID: PMC8518835
- DOI: 10.1111/jth.15439
First evaluation of the safety, pharmacokinetics, and pharmacodynamics of BAY 2433334, a small molecule targeting coagulation factor XIa
Abstract
Background: Coagulation factor XI (FXI) contributes to the development of thrombosis but appears to play a minor role in hemostasis and is, therefore, an attractive anticoagulant drug target.
Objectives: To evaluate the safety, pharmacokinetic, and pharmacodynamic properties of BAY 2433334, an orally administered small molecule targeting activated FXI (FXIa), in healthy men.
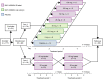
Patients/methods: This phase 1 study was conducted in two parts. In part 1, 70 volunteers were randomized 4:1 to receive a single oral dose of BAY 2433334 (5-150 mg as oral solution or immediate-release tablets) or placebo. In part 2, 16 volunteers received a single oral dose of five BAY 2433334 5-mg tablets with or without a high-calorie breakfast in a randomized crossover study design. Adverse events, pharmacokinetic parameters, and pharmacodynamic parameters were assessed up to 72 h after drug administration. Volunteers were followed up after 7 to 14 days.
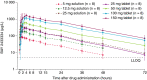
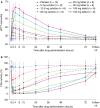
Results: BAY 2433334 demonstrated favorable safety and tolerability with a dose-dependent increase in exposure and a terminal half-life of 14.2 to 17.4 h. A high-calorie breakfast reduced mean maximum plasma concentration and exposure by 31% and 12.4%, respectively. AY 2433334 was associated with a dose-dependent inhibition of FXIa activity and an increase in activated partial thromboplastin time. Bleeding times in volunteers who had received BAY 2433334 were similar to those in volunteers who had received placebo.
Conclusions: These data indicate that BAY 2433334 is a promising development candidate for once-daily oral anticoagulation; it is being evaluated in phase 2 dose-finding studies in patients at risk of thrombosis.
Keywords: anticoagulant; factor XI; hemostasis; phase 1; thrombosis.
© 2021 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
Drs. Thomas, Kanefendt, Schwers, Unger, and Yassen are employees of Bayer AG, which provided funding for this study. Dr. Boxnick is an employee of CRS Clinical Research Services, which received funding from Bayer AG.
Figures

Similar articles
-
Pharmacokinetics, pharmacodynamics and safety of BAY 2433334, a novel activated factor XI inhibitor, in healthy volunteers: A randomized phase 1 multiple-dose study.Br J Clin Pharmacol. 2022 Jul;88(7):3447-3462. doi: 10.1111/bcp.15230. Epub 2022 Mar 24. Br J Clin Pharmacol. 2022. PMID: 35014061 Free PMC article. Clinical Trial.
-
First randomized evaluation of safety, pharmacodynamics, and pharmacokinetics of BAY 1831865, an antibody targeting coagulation factor XI and factor XIa, in healthy men.J Thromb Haemost. 2022 Jul;20(7):1684-1695. doi: 10.1111/jth.15744. Epub 2022 May 20. J Thromb Haemost. 2022. PMID: 35490404 Free PMC article. Clinical Trial.
-
BAY 1213790, a fully human IgG1 antibody targeting coagulation factor XIa: First evaluation of safety, pharmacodynamics, and pharmacokinetics.Res Pract Thromb Haemost. 2019 Feb 14;3(2):242-253. doi: 10.1002/rth2.12186. eCollection 2019 Apr. Res Pract Thromb Haemost. 2019. PMID: 31011708 Free PMC article.
-
Clinical Evaluation of Factor XIa Inhibitor Drugs: JACC Review Topic of the Week.J Am Coll Cardiol. 2023 Feb 28;81(8):771-779. doi: 10.1016/j.jacc.2022.11.057. J Am Coll Cardiol. 2023. PMID: 36813377 Free PMC article. Review.
-
Anticoagulant drugs targeting factor XI/XIa and coagulation tests: we urgently need reliable pharmacodynamic data.J Thromb Haemost. 2025 May;23(5):1464-1468. doi: 10.1016/j.jtha.2025.02.025. Epub 2025 Mar 6. J Thromb Haemost. 2025. PMID: 40056986 Review.
Cited by
-
Determination of the Potential Clinical Benefits of Small Molecule Factor XIa Inhibitors in Arterial Thrombosis.ACS Pharmacol Transl Sci. 2023 Jun 30;6(7):970-981. doi: 10.1021/acsptsci.3c00052. eCollection 2023 Jul 14. ACS Pharmacol Transl Sci. 2023. PMID: 37470020 Free PMC article. Review.
-
Factor XI inhibitors are the novel promising anticoagulants in the treatment of age related thrombotic disease.Front Cardiovasc Med. 2025 May 30;12:1498826. doi: 10.3389/fcvm.2025.1498826. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40520928 Free PMC article. Review.
-
The Safety, Pharmacokinetics, and Pharmacodynamics of SHR2285, an Oral Small Molecule Factor XIa Inhibitor, in Healthy Chinese Volunteers.Clin Drug Investig. 2023 Jun;43(6):435-445. doi: 10.1007/s40261-023-01281-8. Epub 2023 Jun 16. Clin Drug Investig. 2023. PMID: 37326942 Clinical Trial.
-
Factor XI Inhibition for the Prevention of Venous Thromboembolism: An Update on Current Evidence and Future perspectives.Vasc Health Risk Manag. 2022 May 10;18:359-373. doi: 10.2147/VHRM.S331614. eCollection 2022. Vasc Health Risk Manag. 2022. PMID: 35707632 Free PMC article. Review.
-
Sex Matters: Policy on Reporting Sex as a Biological Variable at Research and Practice in Thrombosis and Hemostasis.Res Pract Thromb Haemost. 2023 Nov 7;7(8):102256. doi: 10.1016/j.rpth.2023.102256. eCollection 2023 Nov. Res Pract Thromb Haemost. 2023. PMID: 38053984 Free PMC article. No abstract available.
References
-
- Mackman N, Bergmeier W, Stouffer GA, Weitz JI. Therapeutic strategies for thrombosis: new targets and approaches. Nat Rev Drug Discov. 2020;19:333‐352. - PubMed
-
- Zhang H, Lowenberg EC, Crosby JR, et al. Inhibition of the intrinsic coagulation pathway factor XI by antisense oligonucleotides: a novel antithrombotic strategy with lowered bleeding risk. Blood. 2010;116:4684‐4692. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

