The use of remdesivir for the management of patients with moderate-to-severe COVID-19: a systematic review
- PMID: 34192469
- PMCID: PMC8477583
- DOI: 10.1080/14787210.2021.1949984
The use of remdesivir for the management of patients with moderate-to-severe COVID-19: a systematic review
Abstract
Objective: We systematically reviewed the evidence of published original research to determine the role of remdesivir in the management of patients with COVID-19 and a moderate-to-severe course of illness.
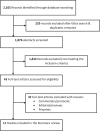
Methods: A systematic search of articles was conducted in scientific databases, with the latest update in May 2021. This paper systematically reviewed the clinical evidence available (randomized controlled trials, compassionate use studies, and case reports) on the use of remdesivir for patients with moderate or severe COVID-19.
Results: A total of eleven studies were included: four studies based on compassionate use of remdesivir, three randomized, double-blind, placebo-controlled, multicentre trials, three randomized, open-label, phase III trials, and one case report. Clinical improvement and mortality rates in patients who used remdesivir varied across studies.
Conclusion: Given the current evidence, there is insufficient data to confidently recommend the use of remdesivir alone for the treatment of adult hospitalized patients with moderate-to-severe COVID-19. However, remdesivir may be considered along with an anti-inflammatory agent in patients with pneumonia, on oxygen support, provided there is close monitoring of clinical and laboratory parameters and adverse events.
Keywords: Adverse events; SARS-CoV-2; critically ill; evidence-based medicine; remdesivir.
References
-
- Abi-Habib MMillions had Risen out of poverty. Coronavirus is pulling them back. The New York Times [Internet]. 2020. Apr 30 [cited 2020 Jun 2]. Available from: https://www.nytimes.com/2020/04/30/world/asia/coronavirus-poverty-unempl...
-
- Murthy S, Gomersall CD, Fowler RA.. Care for critically Ill patients with COVID-19. JAMA. 2020;323(15):1499–1500. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous