Tissue engineered bovine saphenous vein extracellular matrix scaffolds produced via antigen removal achieve high in vivo patency rates
- PMID: 34192567
- PMCID: PMC8542604
- DOI: 10.1016/j.actbio.2021.06.034
Tissue engineered bovine saphenous vein extracellular matrix scaffolds produced via antigen removal achieve high in vivo patency rates
Abstract
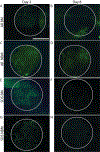
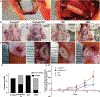
Diseases of small diameter blood vessels encompass the largest portion of cardiovascular diseases, with over 4.2 million people undergoing autologous vascular grafting every year. However, approximately one third of patients are ineligible for autologous vascular grafting due to lack of suitable donor vasculature. Acellular extracellular matrix (ECM) scaffolds derived from xenogeneic vascular tissue have potential to serve as ideal biomaterials for production of off-the-shelf vascular grafts capable of eliminating the need for autologous vessel harvest. A modified antigen removal (AR) tissue process, employing aminosulfabetaine-16 (ASB-16) was used to create off-the-shelf small diameter (< 3 mm) vascular graft from bovine saphenous vein ECM scaffolds with significantly reduced antigenic content, while retaining native vascular ECM protein structure and function. Elimination of native tissue antigen content conferred graft-specific adaptive immune avoidance, while retention of native ECM protein macromolecular structure resulted in pro-regenerative cellular infiltration, ECM turnover and innate immune self-recognition in a rabbit subpannicular model. Finally, retention of the delicate vascular basement membrane protein integrity conferred endothelial cell repopulation and 100% patency rate in a rabbit jugular interposition model, comparable only to Autograft implants. Alternatively, the lack of these important basement membrane proteins in otherwise identical scaffolds yielded a patency rate of only 20%. We conclude that acellular antigen removed bovine saphenous vein ECM scaffolds have potential to serve as ideal off-the-shelf small diameter vascular scaffolds with high in vivo patency rates due to their low antigen content, retained native tissue basement membrane integrity and preserved native ECM structure, composition and functional properties. STATEMENT OF SIGNIFICANCE: The use of autologous vessels for the treatment of small diameter vascular diseases is common practice. However, the use of autologous tissue poses significant complications due to tissue harvest and limited availability. Developing an alternative vessel for use for the treatment of small diameter vessel diseases can potentially increase the success rate of autologous vascular grafting by eliminating complications related to the use of autologous vessel and increased availability. This manuscript demonstrates the potential of non-antigenic extracellular matrix (ECM) scaffolds derived from xenogeneic vascular tissue as off-the-shelf vascular grafts for the treatment of small diameter vascular diseases.
Keywords: Extracellular matrix; Small diameter vessel; Tissue engineering.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Small Diameter Xenogeneic Extracellular Matrix Scaffolds for Vascular Applications.Tissue Eng Part B Rev. 2020 Feb;26(1):26-45. doi: 10.1089/ten.TEB.2019.0229. Epub 2019 Nov 27. Tissue Eng Part B Rev. 2020. PMID: 31663438 Free PMC article. Review.
-
Antigen removal process preserves function of small diameter venous valved conduits, whereas SDS-decellularization results in significant valvular insufficiency.Acta Biomater. 2020 Apr 15;107:115-128. doi: 10.1016/j.actbio.2020.03.003. Epub 2020 Mar 7. Acta Biomater. 2020. PMID: 32151701 Free PMC article.
-
Basement Membrane of Tissue Engineered Extracellular Matrix Scaffolds Modulates Rapid Human Endothelial Cell Recellularization and Promote Quiescent Behavior After Monolayer Formation.Front Bioeng Biotechnol. 2022 Aug 2;10:903907. doi: 10.3389/fbioe.2022.903907. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35983533 Free PMC article.
-
Tissue-engineered blood vessel graft produced by self-derived cells and allogenic acellular matrix: a functional performance and histologic study.Ann Plast Surg. 2009 Mar;62(3):297-303. doi: 10.1097/SAP.0b013e318197eb19. Ann Plast Surg. 2009. PMID: 19240529
-
Tissue engineered small-diameter vascular grafts.Clin Plast Surg. 2003 Oct;30(4):507-17. doi: 10.1016/s0094-1298(03)00069-5. Clin Plast Surg. 2003. PMID: 14621299 Review.
Cited by
-
Biological Materials for Tissue-Engineered Vascular Grafts: Overview of Recent Advancements.Biomolecules. 2023 Sep 14;13(9):1389. doi: 10.3390/biom13091389. Biomolecules. 2023. PMID: 37759789 Free PMC article. Review.
-
Preparation and Use of Decellularized Extracellular Matrix for Tissue Engineering.J Funct Biomater. 2022 Nov 14;13(4):240. doi: 10.3390/jfb13040240. J Funct Biomater. 2022. PMID: 36412881 Free PMC article. Review.
-
Clinical Application for Tissue Engineering Focused on Materials.Biomedicines. 2022 Jun 17;10(6):1439. doi: 10.3390/biomedicines10061439. Biomedicines. 2022. PMID: 35740460 Free PMC article. Review.
-
The application of composite scaffold materials based on decellularized vascular matrix in tissue engineering: a review.Biomed Eng Online. 2023 Jun 19;22(1):62. doi: 10.1186/s12938-023-01120-z. Biomed Eng Online. 2023. PMID: 37337190 Free PMC article. Review.
-
Current Challenges and Future Promise for Use of Extracellular Matrix Scaffold to Achieve the Whole Organ Tissue Engineering Moonshot.Stem Cells Transl Med. 2023 Sep 15;12(9):588-602. doi: 10.1093/stcltm/szad046. Stem Cells Transl Med. 2023. PMID: 37589543 Free PMC article. Review.
References
-
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ, C. American Heart Association Advocacy Coordinating, C. Stroke, R. Council on Cardiovascular, Intervention, C. Council on Clinical, E. Council on, Prevention, A. Council on, Thrombosis, B. Vascular, C. Council on, C. Critical, Perioperative, Resuscitation, N. Council on Cardiovascular, D. Council on the Kidney in Cardiovascular, S. Council on Cardiovascular, Anesthesia, C. Interdisciplinary Council on Quality of, and R. Outcomes, Forecasting the future of cardiovascular disease in the United States: a policy statement from the American heart association, Circulation 123 (8) (2011) 933–944. - PubMed
-
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, E. American Heart Association Council on, C. Prevention Statistics, and S. Stroke Statistics, Heart disease and stroke statistics-2019 update: a report from the American heart association, Circulation 139 (10) (2019) e56–e528. - PubMed
-
- Fryar CD, Chen TC, Li X, Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999–2010, NCHS Data Brief. (103) (2012) 1–8. - PubMed
-
- Alexander JH, Smith PK, Coronary-artery bypass grafting, N. Engl. J. Med. 374 (20) (2016) 1954–1964. - PubMed
-
- Slovut DP, Lipsitz EC, Surgical technique and peripheral artery disease, Circulation 126 (9) (2012) 1127–1138. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

