Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2
- PMID: 34192737
- PMCID: PMC8373615
- DOI: 10.1038/s41586-021-03739-1
Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2
Abstract
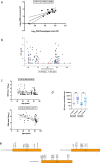
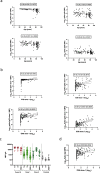
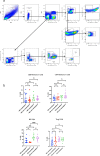
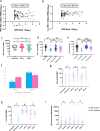
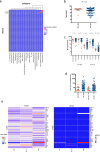
Although two-dose mRNA vaccination provides excellent protection against SARS-CoV-2, there is little information about vaccine efficacy against variants of concern (VOC) in individuals above eighty years of age1. Here we analysed immune responses following vaccination with the BNT162b2 mRNA vaccine2 in elderly participants and younger healthcare workers. Serum neutralization and levels of binding IgG or IgA after the first vaccine dose were lower in older individuals, with a marked drop in participants over eighty years old. Sera from participants above eighty showed lower neutralization potency against the B.1.1.7 (Alpha), B.1.351 (Beta) and P.1. (Gamma) VOC than against the wild-type virus and were more likely to lack any neutralization against VOC following the first dose. However, following the second dose, neutralization against VOC was detectable regardless of age. The frequency of SARS-CoV-2 spike-specific memory B cells was higher in elderly responders (whose serum showed neutralization activity) than in non-responders after the first dose. Elderly participants showed a clear reduction in somatic hypermutation of class-switched cells. The production of interferon-γ and interleukin-2 by SARS-CoV-2 spike-specific T cells was lower in older participants, and both cytokines were secreted primarily by CD4 T cells. We conclude that the elderly are a high-risk population and that specific measures to boost vaccine responses in this population are warranted, particularly where variants of concern are circulating.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

