The potential role of stress and sex steroids in heritable effects of sevoflurane†
- PMID: 34192761
- PMCID: PMC8444702
- DOI: 10.1093/biolre/ioab129
The potential role of stress and sex steroids in heritable effects of sevoflurane†
Abstract
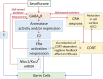
Most surgical procedures require general anesthesia, which is a reversible deep sedation state lacking all perception. The induction of this state is possible because of complex molecular and neuronal network actions of general anesthetics (GAs) and other pharmacological agents. Laboratory and clinical studies indicate that the effects of GAs may not be completely reversible upon anesthesia withdrawal. The long-term neurocognitive effects of GAs, especially when administered at the extremes of ages, are an increasingly recognized health concern and the subject of extensive laboratory and clinical research. Initial studies in rodents suggest that the adverse effects of GAs, whose actions involve enhancement of GABA type A receptor activity (GABAergic GAs), can also extend to future unexposed offspring. Importantly, experimental findings show that GABAergic GAs may induce heritable effects when administered from the early postnatal period to at least young adulthood, covering nearly all age groups that may have children after exposure to anesthesia. More studies are needed to understand when and how the clinical use of GAs in a large and growing population of patients can result in lower resilience to diseases in the even larger population of their unexposed offspring. This minireview is focused on the authors' published results and data in the literature supporting the notion that GABAergic GAs, in particular sevoflurane, may upregulate systemic levels of stress and sex steroids and alter expressions of genes that are essential for the functioning of these steroid systems. The authors hypothesize that stress and sex steroids are involved in the mediation of sex-specific heritable effects of sevoflurane.
Keywords: DNA methylation; GABA type A receptor; K+-2Cl- (KCC2) Cl- exporter; Na+-K+-Cl- (NKCC1) Cl- importer; corticosterone; estradiol; general anesthetic; intergenerational effects; sevoflurane; testosterone.
© The Author(s) 2021. Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
A Methyltransferase Inhibitor (Decitabine) Alleviates Intergenerational Effects of Paternal Neonatal Exposure to Anesthesia With Sevoflurane.Anesth Analg. 2020 Oct;131(4):1291-1299. doi: 10.1213/ANE.0000000000005097. Anesth Analg. 2020. PMID: 32925350 Free PMC article.
-
Roles of Testosterone and Estradiol in Mediation of Acute Neuroendocrine and Electroencephalographic Effects of Sevoflurane During the Sensitive Period in Rats.Front Endocrinol (Lausanne). 2020 Sep 30;11:545973. doi: 10.3389/fendo.2020.545973. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33101193 Free PMC article.
-
Anesthesia with sevoflurane in neonatal rats: Developmental neuroendocrine abnormalities and alleviating effects of the corticosteroid and Cl(-) importer antagonists.Psychoneuroendocrinology. 2015 Oct;60:173-81. doi: 10.1016/j.psyneuen.2015.06.016. Epub 2015 Jun 25. Psychoneuroendocrinology. 2015. PMID: 26150359 Free PMC article.
-
Neuroendocrine, epigenetic, and intergenerational effects of general anesthetics.World J Psychiatry. 2020 May 19;10(5):81-94. doi: 10.5498/wjp.v10.i5.81. eCollection 2020 May 19. World J Psychiatry. 2020. PMID: 32477904 Free PMC article. Review.
-
Prenatal sevoflurane exposure: Effects of iron metabolic dysfunction on offspring cognition and potential mechanism.Int J Dev Neurosci. 2021 Feb;81(1):1-9. doi: 10.1002/jdn.10080. Epub 2020 Dec 14. Int J Dev Neurosci. 2021. PMID: 33259670 Review.
Cited by
-
Neurobehavioral Abnormalities in Offspring of Young Adult Male Rats With a History of Traumatic Brain Injury.J Neurotrauma. 2024 Apr;41(7-8):969-984. doi: 10.1089/neu.2023.0364. Epub 2024 Feb 14. J Neurotrauma. 2024. PMID: 38279844 Free PMC article.
-
Epigenetic Mechanisms of Postoperative Cognitive Impairment Induced by Anesthesia and Neuroinflammation.Cells. 2022 Sep 21;11(19):2954. doi: 10.3390/cells11192954. Cells. 2022. PMID: 36230916 Free PMC article. Review.
References
-
- Letcher P, Greenwood CJ, Romaniuk H, Spry E, Macdonald JA, McAnally H, Thomson KC, Youssef G, Hutchinson D, McIntosh J, Sanson A, Ryan J et al. Adolescent and young adult mental health problems and infant offspring behavior: findings from a prospective intergenerational cohort study. J Affect Disord 2020; 272:521–528. - PubMed

