Aflibercept, a VEGF (Vascular Endothelial Growth Factor)-Trap, Reduces Vascular Permeability and Stroke-Induced Brain Swelling in Obese Mice
- PMID: 34192895
- PMCID: PMC8312568
- DOI: 10.1161/STROKEAHA.121.034362
Aflibercept, a VEGF (Vascular Endothelial Growth Factor)-Trap, Reduces Vascular Permeability and Stroke-Induced Brain Swelling in Obese Mice
Abstract
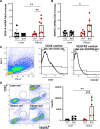
Background and purpose: Brain edema is an important underlying pathology in acute stroke, especially when comorbidities are present. VEGF (Vascular endothelial growth factor) signaling is implicated in edema. This study investigated whether obesity impacts VEGF signaling and brain edema, as well as whether VEGF inhibition alters stroke outcome in obese subjects.
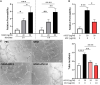
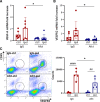
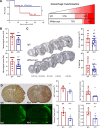
Methods: High-fat diet-induced obese mice were subjected to a transient middle cerebral artery occlusion. VEGF-A and VEGFR2 (receptor) expression, infarct volume, and swelling were measured 3 days post-middle cerebral artery occlusion. To validate the effect of an anti-VEGF strategy, we used aflibercept, a fusion protein that has a VEGF-binding domain and acts as a decoy receptor, in human umbilical vein endothelial cells stimulated with rVEGF (recombinant VEGF; 50 ng/mL) for permeability and tube formation. In vivo, aflibercept (10 mg/kg) or IgG control was administered in obese mice 3 hours after transient 30 minutes middle cerebral artery occlusion. Blood-brain barrier integrity was assessed by IgG staining and dextran extravasation in the postischemic brain. A separate cohort of nonobese (lean) mice was subjected to 40 minutes middle cerebral artery occlusion to test the effect of aflibercept on malignant infarction.
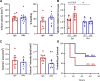
Results: Compared with lean mice, obese mice had increased mortality, infarct volume, swelling, and blood-brain barrier disruption. These outcomes were also associated with increased VEGF-A and VEGFR2 expression. Aflibercept reduced VEGF-A-stimulated permeability and tube formation in human umbilical vein endothelial cells. Compared with the IgG-treated controls, mice treated with aflibercept had reduced mortality rates (40% versus 17%), hemorrhagic transformation (43% versus 27%), and brain swelling (28% versus 18%), although the infarct size was similar. In nonobese mice with large stroke, aflibercept neither improved nor exacerbated stroke outcomes.
Conclusions: The study demonstrates that aflibercept selectively attenuates stroke-induced brain edema and vascular permeability in obese mice. These findings suggest the repurposing of aflibercept to reduce obesity-enhanced brain edema in acute stroke.
Keywords: brain edema; comorbidity; obesity; stroke; vascular endothelial growth factor.
Figures

References
-
- O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006; 59:467–477. doi: 10.1002/ana.20741 - PubMed
-
- Pinto A, Tuttolomondo A, Di Raimondo D, Fernandez P, Licata G. Cerebrovascular risk factors and clinical classification of strokes. Semin Vasc Med. 2004; 4:287–303. doi: 10.1055/s-2004-861497 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

