Pyramiding of cry toxins and methanol producing genes to increase insect resistance in cotton
- PMID: 34193022
- PMCID: PMC8253136
- DOI: 10.1080/21645698.2021.1944013
Pyramiding of cry toxins and methanol producing genes to increase insect resistance in cotton
Abstract
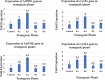
The idea of enhanced methanol production from cell wall by pectin methyl esterase enzymes (PME) combined with expression of cry genes from Bacillus thuringiensis as a strategy to improve insect pest control in cotton is presented. We constructed a cassette containing two cry genes (cry1Fa and Cry32Aa) and two pme genes, one from Arabidopsis thaliana (AtPME), and other from Aspergillus. niger (AnPME) in pCAMBIA1301 plant expression vector using CAMV-35S promoter. This construction was transformed in Eagle-2 cotton variety by using shoot apex-cut Agrobacterium-mediated transformation. Expression of cry genes and pme genes was confirmed by qPCR. Methanol production was measured in control and in the cry and pme transformed plants showing methanol production only in transformed plants, in contrast to the non-transgenic cotton plants. Finally, insect bioassays performed with transgenic plants expressing cry and pme genes showed 100% mortality for Helicoverpa armigera (cotton bollworm) larvae, 70% mortality for Pectinophora gossypiella (pink bollworm) larvae and 95% mortality of Earias fabia, (spotted bollworm) larvae, that was higher than the transgenic plants expressing only cry genes that showed 84%, 49% and 79% mortality, respectively. These results demonstrate that Bt. cry-genes coupled with pme genes are an effective strategy to improve the control of different insect pests.
Keywords: Cotton; agrobacterium; eagle-2; insecticidal cry proteins; pectin methyl esterase enzyme; transformation.
Figures
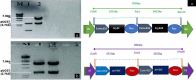


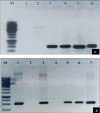


References
-
- Tarazi R, JLS J, MF V.. Biotechnological solutions for major cotton (Gossypium hirsutum) pathogens and pests. Biotech Res and Innovation. 2020;3:19-26..
-
- Junyu L, Zhang S, Xiangzhen Z, Jichao J, Zhang K, Chunyi W, Zhang L, Li W, Jiniie C. Effects of NaCl stress on the biochemical substances in Bt cotton as well as on the growth and development and adult oviposition selectivity of Helicoverpa armigera. Jl of Cotton Res. 2019;2(1):4. doi:10.1186/s42397-019-0020-7. - DOI
-
- Verma C, AKM T, Mishra S. Biochemical and molecular characterization of cell wall degrading enzyme, pectin methylesterase versus banana ripening: an overview. Asian J of Biotech. 2017;9(1):1–23. doi:10.3923/ajbkr.2017.1.23. - DOI
-
- Williams M. Cotton insect losses. Mississippi State University; 1986-2015. 2016.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
