Transfer of Mycoplasma hyopneumoniae-specific cell mediated immunity to neonatal piglets
- PMID: 34193259
- PMCID: PMC8247214
- DOI: 10.1186/s13567-021-00968-0
Transfer of Mycoplasma hyopneumoniae-specific cell mediated immunity to neonatal piglets
Abstract
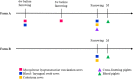
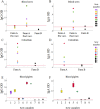
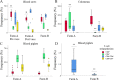
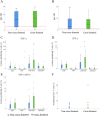
Mycoplasma hyopneumoniae is the primary agent of enzootic pneumonia in pigs. Although cell mediated immunity (CMI) may play a role in protection against M. hyopneumoniae, its transfer from sows to their offspring is poorly characterized. Therefore, maternally-derived CMI was studied in piglets from vaccinated and non-vaccinated sows. The potential influence of cross-fostering before colostrum ingestion on the transfer of CMI from dam to piglets was also investigated. Six M. hyopneumoniae vaccinated sows from an endemically infected herd and 47 of their piglets, of which 24 piglets were cross-fostered, were included, as well as three non-vaccinated control sows from an M. hyopneumoniae-free herd and 24 of their piglets. Vaccinated sows received a commercial bacterin intramuscularly at 6 and 3 weeks prior to farrowing. The TNF-α, IFN-γ and IL-17A production by different T-cell subsets in blood of sows, colostrum and blood of piglets was assessed using a recall assay. In blood of sows cytokine producing T-cells were increased upon M. hyopneumoniae vaccination. Similarly, M. hyopneumoniae-specific T-cells were detected in blood of 2-day-old piglets born from these vaccinated sows. In contrast, no M. hyopneumoniae-specific cytokine producing T-cells were found in blood of piglets from control sows. No difference was found in M. hyopneumoniae-specific CMI between cross-fostered and non-cross-fostered piglets. In conclusion, different M. hyopneumoniae-specific T-cell subsets are transferred from the sow to the offspring. Further studies are required to investigate the role of these transferred cells on immune responses in piglets and their potential protective effect against M. hyopneumoniae infections.
Keywords: Mycoplasma hyopneumoniae; cell mediated immunity; cross-fostering; maternal immunity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Effect of cross-fostering on transfer of maternal immunity to Mycoplasma hyopneumoniae to piglets.Vet Rec. 2011 Jan 29;168(4):100. doi: 10.1136/vr.c6163. Epub 2011 Jan 26. Vet Rec. 2011. PMID: 21493469
-
Passive transfer of maternal Mycoplasma hyopneumoniae-specific cellular immunity to piglets.Clin Vaccine Immunol. 2008 Mar;15(3):540-3. doi: 10.1128/CVI.00466-07. Epub 2008 Jan 9. Clin Vaccine Immunol. 2008. PMID: 18184823 Free PMC article.
-
Antibody response to Mycoplasma hyopneumoniae infection in vaccinated pigs with or without maternal antibodies induced by sow vaccination.J Vet Med B Infect Dis Vet Public Health. 2006 Jun;53(5):229-33. doi: 10.1111/j.1439-0450.2006.00952.x. J Vet Med B Infect Dis Vet Public Health. 2006. PMID: 16732881
-
Mycoplasma hyopneumoniae: from disease to vaccine development.Vet Microbiol. 2013 Aug 30;165(3-4):234-42. doi: 10.1016/j.vetmic.2013.04.019. Epub 2013 Apr 24. Vet Microbiol. 2013. PMID: 23680109 Review.
-
Perspectives for improvement of Mycoplasma hyopneumoniae vaccines in pigs.Vet Res. 2021 May 8;52(1):67. doi: 10.1186/s13567-021-00941-x. Vet Res. 2021. PMID: 33964969 Free PMC article. Review.
Cited by
-
Cross-sectional study of Mycoplasma hyopharyngis, Mycoplasma hyopneumoniae, Mycoplasma hyorhinis and Mycoplasma hyosynoviae in the tonsils of fattening pigs from Central-Eastern Europe.Porcine Health Manag. 2025 Mar 6;11(1):11. doi: 10.1186/s40813-025-00429-6. Porcine Health Manag. 2025. PMID: 40051016 Free PMC article.
-
Different local, innate and adaptive immune responses are induced by two commercial Mycoplasma hyopneumoniae bacterins and an adjuvant alone.Front Immunol. 2022 Dec 7;13:1015525. doi: 10.3389/fimmu.2022.1015525. eCollection 2022. Front Immunol. 2022. PMID: 36569943 Free PMC article.
-
Impact of maternally derived immunity on immune responses elicited by piglet early vaccination against the most common pathogens involved in porcine respiratory disease complex.Porcine Health Manag. 2022 Mar 16;8(1):11. doi: 10.1186/s40813-022-00252-3. Porcine Health Manag. 2022. PMID: 35296365 Free PMC article. Review.
-
Long-term follow-up of Mycoplasma hyopneumoniae-specific immunity in vaccinated pigs.Vet Res. 2023 Mar 1;54(1):16. doi: 10.1186/s13567-023-01145-1. Vet Res. 2023. PMID: 36859402 Free PMC article.
References
-
- Pieters M, Maes D. Mycoplasmosis. In: Zimmermann JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, Zhang J, editors. Diseases of swine, Edition 11. New York: Wiley; 2019.
-
- Rycroft A. The general characteristics and classification of porcine Mycoplasma species. In: Maes D, Sibila M, Pieters M, editors. Mycoplasmas in Swine. Belgium: Acco; 2020.
-
- Sibila M, Bernal R, Torrents D, Riera P, Llopart D, Calsamiglia M, Segales J. Effect of sow vaccination against Mycoplamsa hyopneumoniae on sow and piglets colonization and seroconversion, and pig lung lesions at slaughter. Vet Microbiol. 2008;127:165–170. doi: 10.1016/j.vetmic.2007.07.027. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

