'Give Us The Tools!': development of knowledge transfer tools to support the involvement of patient partners in the development of clinical trial protocols with patient-reported outcomes (PROs), in accordance with SPIRIT-PRO Extension
- PMID: 34193492
- PMCID: PMC8246365
- DOI: 10.1136/bmjopen-2020-046450
'Give Us The Tools!': development of knowledge transfer tools to support the involvement of patient partners in the development of clinical trial protocols with patient-reported outcomes (PROs), in accordance with SPIRIT-PRO Extension
Abstract
Objectives: (a) To adapt the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT)-patient-reported outcome (PRO) Extension guidance to a user-friendly format for patient partners and (b) to codesign a web-based tool to support the dissemination and uptake of the SPIRIT-PRO Extension by patient partners.
Design: A 1-day patient and public involvement session.
Participants: Seven patient partners.
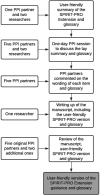
Methods: A patient partner produced an initial lay summary of the SPIRIT-PRO guideline and a glossary. We held a 1-day PPI session in November 2019 at the University of Birmingham. Five patient partners discussed the draft lay summary, agreed on the final wording, codesigned and agreed the final content for both tools. Two additional patient partners were involved in writing the manuscript. The study compiled with INVOLVE guidelines and was reported according to the Guidance for Reporting Involvement of Patients and the Public 2 checklist.
Results: Two user-friendly tools were developed to help patients and members of the public be involved in the codesign of clinical trials collecting PROs. The first tool presents a lay version of the SPIRIT-PRO Extension guidance. The second depicts the most relevant points, identified by the patient partners, of the guidance through an interactive flow diagram.
Conclusions: These tools have the potential to support the involvement of patient partners in making informed contributions to the development of PRO aspects of clinical trial protocols, in accordance with the SPIRIT-PRO Extension guidelines. The involvement of patient partners ensured the tools focused on issues most relevant to them.
Keywords: protocols & guidelines; qualitative research; quality in health care.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MC and AS receive funding from the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre, the NIHR Surgical Reconstruction and Microbiology Research Centre and NIHR ARC West Midlands at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Health Data Research UK, Innovate UK (part of UK Research and Innovation), Macmillan Cancer Support, UCB Pharma. The views expressed in this article are those of the author(s) and not necessarily those of the NIHR, or the Department of Health and Social Care. MC has received personal fees from Astellas, Takeda, Merck, Daiichi Sankyo, Glaukos, GlaxoSmithKline and the Patient-Centered Outcomes Research Institute (PCORI) outside the submitted work. RM-B is supported by the Australian Government by a National Health and Medical Research. OLA declares personal fees from Gilead Sciences Ltd and GlaxoSmithKline outside the submitted work.
Figures

References
-
- FDA . Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims., 2009. Available: http://wwwfdagov/downloads/Drugs/GuidanceComplianceRegulatoryInformation... - PMC - PubMed
-
- UK Clinical Research Collaboration . Understanding clinical trials 2006. Available: https://www.ukcrc.org/wp-content/uploads/2014/03/iCT_Booklet.pdf
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
