Corticotropin-Releasing Hormone from the Pontine Micturition Center Plays an Inhibitory Role in Micturition
- PMID: 34193553
- PMCID: PMC8387110
- DOI: 10.1523/JNEUROSCI.0684-21.2021
Corticotropin-Releasing Hormone from the Pontine Micturition Center Plays an Inhibitory Role in Micturition
Abstract
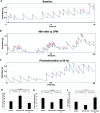
Lower urinary tract or voiding disorders are prevalent across all ages and affect >40% of adults over 40 years old, leading to decreased quality of life and high health care costs. The pontine micturition center (PMC; i.e., Barrington's nucleus) contains a large population of neurons that localize the stress-related neuropeptide, corticotropin-releasing hormone (CRH) and project to neurons in the spinal cord to regulate micturition. How the PMC and CRH-expressing neurons in the PMC control volitional micturition is of critical importance for human voiding disorders. To investigate the specific role of CRH in the PMC, neurons in the PMC-expressing CRH were optogenetically activated during in vivo cystometry in unanesthetized mice of either sex. Optogenetic activation of CRH-PMC neurons led to increased intermicturition interval and voided volume, similar to the altered voiding phenotype produced by social stress. Female mice showed a significantly more pronounced phenotype change compared with male mice. These effects were eliminated by CRH-receptor 1 antagonist pretreatment. Optogenetic inhibition of CRH-PMC neurons led to an altered voiding phenotype characterized by more frequent voids and smaller voided volumes. Last, in a cyclophosphamide cystitis model of bladder overactivity, optogenetic activation of CRH-PMC neurons returned the voiding pattern to normal. Collectively, our findings demonstrate that CRH from PMC spinal-projecting neurons has an inhibitory function on micturition and is a potential therapeutic target for human disease states, such as voiding postponement, urinary retention, and underactive or overactive bladder.SIGNIFICANCE STATEMENT The pontine micturition center (PMC), which is a major regulator of volitional micturition, is neurochemically heterogeneous, and excitatory neurotransmission derived from PMC neurons is thought to mediate the micturition reflex. In the present study, using optogenetic manipulation of CRH-containing neurons in double-transgenic mice, we demonstrate that CRH, which is prominent in PMC-spinal projections, has an inhibitory function on volitional micturition. Moreover, engaging this inhibitory function of CRH can ameliorate bladder hyperexcitability induced by cyclophosphamide in a model of cystitis. The data underscore CRH as a novel target for the treatment of voiding dysfunctions, which are highly prevalent disease processes in children and adults.
Keywords: Barrington's nucleus; lower urinary tract symptoms; micturition; neuro-urology; optogenetics; pontine micturition center.
Copyright © 2021 the authors.
Figures

Similar articles
-
Non-Crh Glutamatergic Neurons in Barrington's Nucleus Control Micturition via Glutamatergic Afferents from the Midbrain and Hypothalamus.Curr Biol. 2019 Sep 9;29(17):2775-2789.e7. doi: 10.1016/j.cub.2019.07.009. Epub 2019 Aug 15. Curr Biol. 2019. PMID: 31422881 Free PMC article.
-
Barrington's nucleus: Neuroanatomic landscape of the mouse "pontine micturition center".J Comp Neurol. 2017 Jul 1;525(10):2287-2309. doi: 10.1002/cne.24215. Epub 2017 Apr 18. J Comp Neurol. 2017. PMID: 28340519 Free PMC article.
-
Probabilistic, spinally-gated control of bladder pressure and autonomous micturition by Barrington's nucleus CRH neurons.Elife. 2020 Apr 29;9:e56605. doi: 10.7554/eLife.56605. Elife. 2020. PMID: 32347794 Free PMC article.
-
Role of Barrington's nucleus in micturition.J Comp Neurol. 2005 Dec 5;493(1):21-6. doi: 10.1002/cne.20719. J Comp Neurol. 2005. PMID: 16255005 Review.
-
Central nervous control of micturition and urine storage.J Smooth Muscle Res. 2005 Jun;41(3):117-32. doi: 10.1540/jsmr.41.117. J Smooth Muscle Res. 2005. PMID: 16006745 Review.
Cited by
-
Expression of Acid-Sensing Ion Channel 3 in Afferents Averts Long-Term Sensitization and the Development of Visceral Pain.Int J Mol Sci. 2024 Nov 21;25(23):12503. doi: 10.3390/ijms252312503. Int J Mol Sci. 2024. PMID: 39684215 Free PMC article.
-
Male Lower Urinary Tract Dysfunction: An Underrepresented Endpoint in Toxicology Research.Toxics. 2022 Feb 16;10(2):89. doi: 10.3390/toxics10020089. Toxics. 2022. PMID: 35202275 Free PMC article. Review.
-
Full regeneration of descending corticotropin-releasing hormone axons after a complete spinal cord injury in lampreys.Comput Struct Biotechnol J. 2022 Oct 18;20:5690-5697. doi: 10.1016/j.csbj.2022.10.020. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36320936 Free PMC article.
-
Should We Be Treating Affective Symptoms, Like Anxiety and Depression Which May Be Related to LUTD in Patients With OAB? ICI-RS 2024.Neurourol Urodyn. 2025 Mar;44(3):661-667. doi: 10.1002/nau.25662. Epub 2025 Jan 16. Neurourol Urodyn. 2025. PMID: 39822015 Free PMC article. Review.
-
Somatostatin Neurons in the Mouse Pontine Nucleus Activate GABAA Receptor Mediated Synaptic Currents in Locus Coeruleus Neurons.Front Synaptic Neurosci. 2021 Oct 4;13:754786. doi: 10.3389/fnsyn.2021.754786. eCollection 2021. Front Synaptic Neurosci. 2021. PMID: 34675794 Free PMC article.
References
-
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ (2010) Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 15:896–904. 10.1038/mp.2010.66 - DOI - PMC - PubMed
-
- Barrington FJ (1925) The effect of lesions of the hind- and mid-brain on micturition in the cat. Exp Physiol 15:81–102. 10.1113/expphysiol.1925.sp000345 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
