Gene therapy knockdown of Hippo signaling induces cardiomyocyte renewal in pigs after myocardial infarction
- PMID: 34193613
- PMCID: PMC9476348
- DOI: 10.1126/scitranslmed.abd6892
Gene therapy knockdown of Hippo signaling induces cardiomyocyte renewal in pigs after myocardial infarction
Abstract
Human heart failure, a leading cause of death worldwide, is a prominent example of a chronic disease that may result from poor cell renewal. The Hippo signaling pathway is an inhibitory kinase cascade that represses adult heart muscle cell (cardiomyocyte) proliferation and renewal after myocardial infarction in genetically modified mice. Here, we investigated an adeno-associated virus 9 (AAV9)-based gene therapy to locally knock down the Hippo pathway gene Salvador (Sav) in border zone cardiomyocytes in a pig model of ischemia/reperfusion-induced myocardial infarction. Two weeks after myocardial infarction, when pigs had left ventricular systolic dysfunction, we administered AAV9-Sav-short hairpin RNA (shRNA) or a control AAV9 viral vector carrying green fluorescent protein (GFP) directly into border zone cardiomyocytes via catheter-mediated subendocardial injection. Three months after injection, pig hearts treated with a high dose of AAV9-Sav-shRNA exhibited a 14.3% improvement in ejection fraction (a measure of left ventricular systolic function), evidence of cardiomyocyte division, and reduced scar sizes compared to pigs receiving AAV9-GFP. AAV9-Sav-shRNA-treated pig hearts also displayed increased capillary density and reduced cardiomyocyte ploidy. AAV9-Sav-shRNA gene therapy was well tolerated and did not induce mortality. In addition, liver and lung pathology revealed no tumor formation. Local delivery of AAV9-Sav-shRNA gene therapy to border zone cardiomyocytes in pig hearts after myocardial infarction resulted in tissue renewal and improved function and may have utility in treating heart failure.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


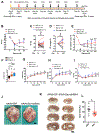
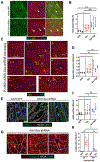
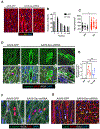
Comment in
-
Cost-effective benefit of iron-replacement therapy.Nat Rev Cardiol. 2021 Sep;18(9):611. doi: 10.1038/s41569-021-00601-9. Nat Rev Cardiol. 2021. PMID: 34244680 No abstract available.
-
Gene therapy against Hippo triggers cardiomyocyte renewal after MI.Nat Rev Cardiol. 2021 Sep;18(9):611. doi: 10.1038/s41569-021-00602-8. Nat Rev Cardiol. 2021. PMID: 34257416 No abstract available.
References
-
- Leach JP, Martin JF, Cardiomyocyte proliferation for therapeutic regeneration. Curr. Cardiol. Rep 20, 63 (2018). - PubMed
-
- Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, dos Remedios C, Malm T, Andrä M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisén J, Dynamics of cell generation and turnover in the human heart. Cell 161, 1566–1575 (2015). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

