Somatic inhibition by microscopic magnetic stimulation
- PMID: 34193906
- PMCID: PMC8245477
- DOI: 10.1038/s41598-021-93114-x
Somatic inhibition by microscopic magnetic stimulation
Abstract
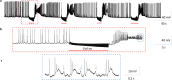
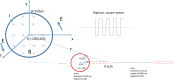
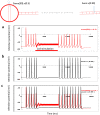
Electric currents can produce quick, reversible control of neural activity. Externally applied electric currents have been used in inhibiting certain ganglion cells in clinical practices. Via electromagnetic induction, a miniature-sized magnetic coil could provide focal stimulation to the ganglion neurons. Here we report that high-frequency stimulation with the miniature coil could reversibly block ganglion cell activity in marine mollusk Aplysia californica, regardless the firing frequency of the neurons, or concentration of potassium ions around the ganglion neurons. Presence of the ganglion sheath has minimal impact on the inhibitory effects of the coil. The inhibitory effect was local to the soma, and was sufficient in blocking the neuron's functional output. Biophysical modeling confirmed that the miniature coil induced a sufficient electric field in the vicinity of the targeted soma. Using a multi-compartment model of Aplysia ganglion neuron, we found that the high-frequency magnetic stimuli altered the ion channel dynamics that were essential for the sustained firing of action potentials in the soma. Results from this study produces several critical insights to further developing the miniature coil technology for neural control by targeting ganglion cells. The miniature coil provides an interesting neural modulation strategy in clinical applications and laboratory research.
Conflict of interest statement
The authors declare no competing interests.
Figures








Similar articles
-
Cellular mechanisms underlying carry-over effects after magnetic stimulation.Sci Rep. 2024 Mar 2;14(1):5167. doi: 10.1038/s41598-024-55915-8. Sci Rep. 2024. PMID: 38431662 Free PMC article.
-
Axonal blockage with microscopic magnetic stimulation.Sci Rep. 2020 Oct 22;10(1):18030. doi: 10.1038/s41598-020-74891-3. Sci Rep. 2020. PMID: 33093520 Free PMC article.
-
Rapid amplification and facilitation of mechanosensory discharge in Aplysia by noxious stimulation.J Neurophysiol. 1993 Sep;70(3):1181-94. doi: 10.1152/jn.1993.70.3.1181. J Neurophysiol. 1993. PMID: 8229167
-
Feeding neural networks in the mollusc Aplysia.Neurosignals. 2004 Jan-Apr;13(1-2):70-86. doi: 10.1159/000076159. Neurosignals. 2004. PMID: 15004426 Review.
-
Voltage-sensitive dyes for monitoring multineuronal activity in the intact central nervous system.Histochem J. 1998 Mar;30(3):169-87. doi: 10.1023/a:1003295319615. Histochem J. 1998. PMID: 10188925 Review.
Cited by
-
Restore axonal conductance in a locally demyelinated axon with electromagnetic stimulation.J Neural Eng. 2025 Feb 14;22(1):016042. doi: 10.1088/1741-2552/adb213. J Neural Eng. 2025. PMID: 39904055 Free PMC article.
-
Cellular mechanisms underlying carry-over effects after magnetic stimulation.Sci Rep. 2024 Mar 2;14(1):5167. doi: 10.1038/s41598-024-55915-8. Sci Rep. 2024. PMID: 38431662 Free PMC article.
-
Neuron matters: neuromodulation with electromagnetic stimulation must consider neurons as dynamic identities.J Neuroeng Rehabil. 2022 Nov 3;19(1):116. doi: 10.1186/s12984-022-01094-4. J Neuroeng Rehabil. 2022. PMID: 36329492 Free PMC article. Review.
-
Micro-Coil Neuromodulation at Single-Cell and Circuit Levels for Inhibiting Natural Neuroactivity, Neutralizing Electric Neural Excitation, and Suppressing Seizures.Adv Sci (Weinh). 2025 Jun;12(22):e2416771. doi: 10.1002/advs.202416771. Epub 2025 Apr 17. Adv Sci (Weinh). 2025. PMID: 40243111 Free PMC article.
-
Modulation of Proteinoid Electrical Spiking Activity with Magnetic Nanoparticles.Langmuir. 2025 Jun 10;41(22):13974-13992. doi: 10.1021/acs.langmuir.5c00932. Epub 2025 May 29. Langmuir. 2025. PMID: 40443122 Free PMC article.
References
-
- Habbema L, Neumann HA. Lidocaine: Local anaesthetic with systemic toxicity. Ned. Tijdschr. Geneeskd. 2008;152:1397. - PubMed
-
- Bhadra, N. & Kilgore, K. L. High-frequency nerve conduction block. in Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference, Vol. 7, 4729–4732. 10.1109/IEMBS.2004.1404309 (2004). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

