Targeting oxidative stress in disease: promise and limitations of antioxidant therapy
- PMID: 34194012
- PMCID: PMC8243062
- DOI: 10.1038/s41573-021-00233-1
Targeting oxidative stress in disease: promise and limitations of antioxidant therapy
Erratum in
-
Author Correction: Targeting oxidative stress in disease: promise and limitations of antioxidant therapy.Nat Rev Drug Discov. 2021 Aug;20(8):652. doi: 10.1038/s41573-021-00267-5. Nat Rev Drug Discov. 2021. PMID: 34257433 Free PMC article. No abstract available.
Abstract
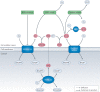
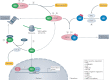
Oxidative stress is a component of many diseases, including atherosclerosis, chronic obstructive pulmonary disease, Alzheimer disease and cancer. Although numerous small molecules evaluated as antioxidants have exhibited therapeutic potential in preclinical studies, clinical trial results have been disappointing. A greater understanding of the mechanisms through which antioxidants act and where and when they are effective may provide a rational approach that leads to greater pharmacological success. Here, we review the relationships between oxidative stress, redox signalling and disease, the mechanisms through which oxidative stress can contribute to pathology, how antioxidant defences work, what limits their effectiveness and how antioxidant defences can be increased through physiological signalling, dietary components and potential pharmaceutical intervention.
© 2021. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Sies, H. Oxidative Stress (ed. Sies, H.) 1–8 (Academic Press, 1985). This article introduces the concept of oxidative stress.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

