Two chemoattenuated PfSPZ malaria vaccines induce sterile hepatic immunity
- PMID: 34194041
- PMCID: PMC11127244
- DOI: 10.1038/s41586-021-03684-z
Two chemoattenuated PfSPZ malaria vaccines induce sterile hepatic immunity
Abstract
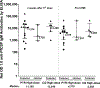
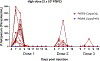
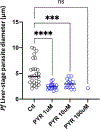
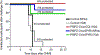
The global decline in malaria has stalled1, emphasizing the need for vaccines that induce durable sterilizing immunity. Here we optimized regimens for chemoprophylaxis vaccination (CVac), for which aseptic, purified, cryopreserved, infectious Plasmodium falciparum sporozoites (PfSPZ) were inoculated under prophylactic cover with pyrimethamine (PYR) (Sanaria PfSPZ-CVac(PYR)) or chloroquine (CQ) (PfSPZ-CVac(CQ))-which kill liver-stage and blood-stage parasites, respectively-and we assessed vaccine efficacy against homologous (that is, the same strain as the vaccine) and heterologous (a different strain) controlled human malaria infection (CHMI) three months after immunization ( https://clinicaltrials.gov/ , NCT02511054 and NCT03083847). We report that a fourfold increase in the dose of PfSPZ-CVac(PYR) from 5.12 × 104 to 2 × 105 PfSPZs transformed a minimal vaccine efficacy (low dose, two out of nine (22.2%) participants protected against homologous CHMI), to a high-level vaccine efficacy with seven out of eight (87.5%) individuals protected against homologous and seven out of nine (77.8%) protected against heterologous CHMI. Increased protection was associated with Vδ2 γδ T cell and antibody responses. At the higher dose, PfSPZ-CVac(CQ) protected six out of six (100%) participants against heterologous CHMI three months after immunization. All homologous (four out of four) and heterologous (eight out of eight) infectivity control participants showed parasitaemia. PfSPZ-CVac(CQ) and PfSPZ-CVac(PYR) induced a durable, sterile vaccine efficacy against a heterologous South American strain of P. falciparum, which has a genome and predicted CD8 T cell immunome that differs more strongly from the African vaccine strain than other analysed African P. falciparum strains.
Conflict of interest statement
Competing interests T.M., L.W.P.C., A.M., A.G., B.K.L.S., N.K.C., S.C., P.F.B., E.R.J., T.L.R. and S.L.H. are salaried, full-time employees of Sanaria, the developer and sponsor of Sanaria PfSPZ Vaccine. S.L.H. and B.K.L.S. also have financial interests in Sanaria. B.K.L.S. and S.L.H. are inventors on patents and patent applications that have been assigned to Sanaria. All other authors declare no competing interests.
Figures
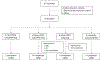
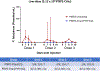
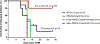

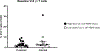


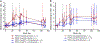
Comment in
-
Malaria vaccine gets a parasite boost in the liver.Nature. 2021 Jul;595(7866):173-174. doi: 10.1038/d41586-021-01720-6. Nature. 2021. PMID: 34193990 No abstract available.
References
-
- WHO. World Malaria Report 2019 (World Health Organization, 2019).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

