Diversity of Marine Macro-Algicolous Endophytic Fungi and Cytotoxic Potential of Biscogniauxia petrensis Metabolites Against Cancer Cell Lines
- PMID: 34194402
- PMCID: PMC8236939
- DOI: 10.3389/fmicb.2021.650177
Diversity of Marine Macro-Algicolous Endophytic Fungi and Cytotoxic Potential of Biscogniauxia petrensis Metabolites Against Cancer Cell Lines
Abstract
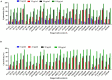
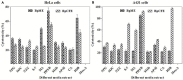
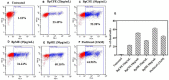
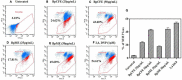
Hypersaline environments are known to support diverse fungal species from various orders. The production of secondary metabolites is one of the strategies that fungi adopt to thrive under such extreme environments, bringing up the stress tolerance response. Some such unique secondary metabolites also exhibit clinical significance. The increasing prevalence of drug resistance in cancer therapy demands further exploration of these novel bioactive compounds as cancer therapeutics. In the present study, a total of 31 endophytic fungi harboring inside red, green, and brown marine algae have been isolated and identified. The maximum likelihood analysis and diversity indices of fungal endophytes revealed the phylogenetic relationship and species richness. The genus Aspergillus was found to be the dominating fungus, followed by Cladosporium spp. All the isolated endophytic fungal extracts were tested for their cytotoxicity against HeLa and A431 cancer cell lines. Nine isolates were further analyzed for their cytotoxic activity from the culture filtrate and mycelia extract. Among these isolates, Biscogniauxia petrensis showed potential cytotoxicity with CC50 values of 18.04 and 24.85 μg/ml against HeLa and A431 cells, respectively. Furthermore, the media and solvent extraction optimization revealed the highest cytotoxic active compounds in ethyl acetate extract from the potato dextrose yeast extract broth medium. The compound-induced cell death via apoptosis was 50-60 and 45% when assayed using propidium iodide-live/dead and loss of mitochondrial membrane potential assay, respectively, in HeLa cells. Four bioactive fractions (bioassay-based) were obtained and analyzed using chromatography and spectroscopy. This study reports, for the first time, the cytotoxic activity of an endophytic fungal community that was isolated from marine macro-algae in the Rameswaram coastal region of Tamil Nadu, India. In addition, B. petrensis is a prominent apoptotic agent, which can be used in pharmaceutical applications as a therapeutic.
Keywords: Biscogniauxia petrensis; algicolous fungi; biodiversity; cytotoxicity; secondary metabolites.
Copyright © 2021 Sahoo, Subban and Chelliah.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Ahsan T., Chen J., Zhao X., Irfan M., Wu Y. (2017). Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by Streptomyces strain KX852460 for the biological control of Rhizoctonia solani AG-3 strain KX852461 to control target spot disease in tobacco leaf. AMB Express 7:54. 10.1186/s13568-017-0351-z - DOI - PMC - PubMed
-
- Arora D., Sharma N., Singamaneni V., Sharma V., Kushwaha M., Abrol V., et al. (2016). Isolation and characterization of bioactive metabolites from Xylaria psidii, an endophytic fungus of the medicinal plant Aegle marmelos and their role in mitochondrial dependent apoptosis against pancreatic cancer cells. Phytomedicine 23 1312–1320. 10.1016/j.phymed.2016.07.004 - DOI - PubMed
-
- Bhagyaraj I., Kunchithapatham V. R. (2016). Diversity and distribution of seaweeds in the shores and water lagoons of Chennai and Rameshwaram coastal areas, South Eastern coast of India. Biodivers. J. 7 923–934.
LinkOut - more resources
Full Text Sources
Miscellaneous

