Expression of ACE2, Soluble ACE2, Angiotensin I, Angiotensin II and Angiotensin-(1-7) Is Modulated in COVID-19 Patients
- PMID: 34194422
- PMCID: PMC8236950
- DOI: 10.3389/fimmu.2021.625732
Expression of ACE2, Soluble ACE2, Angiotensin I, Angiotensin II and Angiotensin-(1-7) Is Modulated in COVID-19 Patients
Abstract
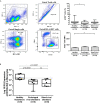
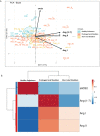
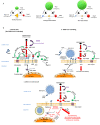
The etiological agent of COVID-19 SARS-CoV-2, is primarily a pulmonary-tropic coronavirus. Infection of alveolar pneumocytes by SARS-CoV-2 requires virus binding to the angiotensin I converting enzyme 2 (ACE2) monocarboxypeptidase. ACE2, present on the surface of many cell types, is known to be a regulator of blood pressure homeostasis through its ability to catalyze the proteolysis of Angiotensin II (Ang II) into Angiotensin-(1-7) [Ang-(1-7)]. We therefore hypothesized that SARS-CoV-2 could trigger variations of ACE2 expression and Ang II plasma concentration in SARS-CoV-2-infected patients. We report here, that circulating blood cells from COVID-19 patients express less ACE2 mRNA than cells from healthy volunteers. At the level of circulating cells, this ACE2 gene dysregulation mainly affects the monocytes, which also show a lower expression of membrane ACE2 protein. Moreover, soluble ACE2 (sACE2) plasma concentrations are lower in prolonged viral shedders than in healthy controls, while the concentration of sACE2 returns to normal levels in short viral shedders. In the plasma of prolonged viral shedders, we also found higher concentrations of Ang II and angiotensin I (Ang I). On the other hand, the plasma levels of Ang-(1-7) remains almost stable in prolonged viral shedders but seems insufficient to prevent the adverse effects of Ang II accumulation. Altogether, these data evidence that the SARS-CoV-2 may affect the expression of blood pressure regulators with possible harmful consequences on COVID-19 outcome.
Keywords: ACE2 (Angiotensin Converting Enzyme-2); COVID-19; Hypertension; Renin – Angiotensin – Aldosterone System; SARS – CoV – 2.
Copyright © 2021 Osman, Melenotte, Brouqui, Million, Lagier, Parola, Stein, La Scola, Meddeb, Mege, Raoult and Devaux.
Conflict of interest statement
CD declares owning Sanofi and Merck shares. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

