Potent SARS-CoV-2-Specific T Cell Immunity and Low Anaphylatoxin Levels Correlate With Mild Disease Progression in COVID-19 Patients
- PMID: 34194438
- PMCID: PMC8237940
- DOI: 10.3389/fimmu.2021.684014
Potent SARS-CoV-2-Specific T Cell Immunity and Low Anaphylatoxin Levels Correlate With Mild Disease Progression in COVID-19 Patients
Abstract
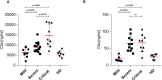
T cells play a fundamental role in the early control and clearance of many viral infections of the respiratory system. In SARS-CoV-2-infected individuals, lymphopenia with drastically reduced CD4+ and CD8+ T cells correlates with Coronavirus disease 2019 (COVID-19)-associated disease severity and mortality. In this study, we characterized cellular and humoral immune responses induced in patients with mild, severe and critical COVID-19. Peripheral blood mononuclear cells of 37 patients with mild, severe and critical COVID-19 and 10 healthy individuals were analyzed by IFNγ ELISpot and multi-color flow cytometry upon stimulation with peptide pools covering complete immunodominant SARS-CoV-2 matrix, nucleocapsid and spike proteins. In addition SARS-CoV-2 antibody levels, neutralization abilities and anaphylatoxin levels were evaluated by various commercially available ELISA platforms. Our data clearly demonstrates a significantly stronger induction of SARS-CoV-2 specific CD8+ T lymphocytes and higher IFNγ production in patients with mild compared to patients with severe or critical COVID-19. In all patients SARS-CoV-2-specific antibodies with similar neutralizing activity were detected, but highest titers of total IgGs were observed in critical patients. Finally, elevated anaphylatoxin C3a and C5a levels were identified in severe and critical COVID-19 patients probably caused by aberrant immune complex formation due to elevated antibody titers in these patients. Crucially, we provide a full picture of cellular and humoral immune responses of COVID-19 patients and prove that robust polyfunctional CD8+ T cell responses concomitant with low anaphylatoxin levels correlate with mild infections. In addition, our data indicates that high SARS-CoV-2 antibody titers are associated with severe disease progression.
Keywords: COVID-19; ELISpot assay; SARS-CoV-2; T cell immunity; anaphylatoxin; neutralizing Abs.
Copyright © 2021 Lafon, Diem, Witting, Zaderer, Bellmann-Weiler, Reindl, Bauer, Griesmacher, Fux, Hoermann, Miller, Zabernigg, Wöll, Wilflingseder, Lass-Flörl and Posch.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
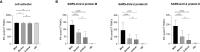

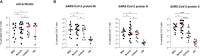

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

