Estimation of the Motor Threshold for Near-Rectangular Stimuli Using the Hodgkin-Huxley Model
- PMID: 34194485
- PMCID: PMC8184325
- DOI: 10.1155/2021/4716161
Estimation of the Motor Threshold for Near-Rectangular Stimuli Using the Hodgkin-Huxley Model
Abstract
The motor threshold measurement is a standard in preintervention probing in TMS experiments. We aim to predict the motor threshold for near-rectangular stimuli to efficiently determine the motor threshold size before any experiments take place. Estimating the behavior of large-scale networks requires dynamically accurate and efficient modeling. We utilized a Hodgkin-Huxley (HH) type model to evaluate motor threshold values and computationally validated its function with known true threshold data from 50 participants trials from state-of-the-art published datasets. For monophasic, bidirectional, and unidirectional rectangular stimuli in posterior-anterior or anterior-posterior directions as generated by the cTMS device, computational modeling of the HH model captured the experimentally measured population-averaged motor threshold values at high precision (maximum error ≤ 8%). The convergence of our biophysically based modeling study with experimental data in humans reveals that the effect of the stimulus shape is strongly correlated with the activation kinetics of the voltage-gated ion channels. The proposed method can reliably predict motor threshold size using the conductance-based neuronal models and could therefore be embedded in new generation neurostimulators. Advancements in neural modeling will make it possible to enhance treatment procedures by reducing the number of delivered magnetic stimuli to participants.
Copyright © 2021 Majid Memarian Sorkhabi et al.
Conflict of interest statement
The authors declared that they have no conflicts of interest to this work.
Figures
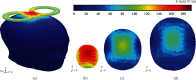
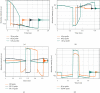
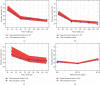

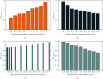
References
-
- Rossini P. M., Burke D., Chen R., et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clinical Neurophysiology. 2015;126(6):1071–1107. doi: 10.1016/j.clinph.2015.02.001. - DOI - PMC - PubMed
-
- Sorkhabi M. M., Frounchi J., Shahabi P., Veladi H. Deep-brain transcranial stimulation: a novel approach for high 3-D resolution. IEEE Access. 2017;5:3157–3171. doi: 10.1109/ACCESS.2017.2672566. - DOI
-
- Sorkhabi M. M., Wendt K., Wilson M. T., Denison T. Numerical modeling of plasticity induced by quadri-pulse stimulation. IEEE Access. 2021;9:26484–26490. doi: 10.1109/access.2021.3057829. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

